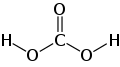
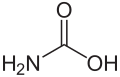
Guanidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Guanidine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 5 N 3 | ||||||||||||||||||
| Brief description |
colorless, hygroscopic crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 59.07 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
50 ° C |
||||||||||||||||||
| solubility |
Easily soluble in ethanol , protonated in water to form readily soluble guanidinium |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−56.0 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Guanidine is a chemical compound at the border between inorganic and organic chemistry . Guanidine can be understood as a nitrogen analogue of carbonic acid . The substance is one of the strongest organic bases and reacts spontaneously in the air with humidity and carbon dioxide to form guanidinium carbonate .
Guanidine was synthesized for the first time in the middle of the 19th century, but the crystal structure could not be clarified until 2009.
Guanidine has been investigated in various quantum chemical calculations , particularly in relation to the concept of Y aromaticity . Furthermore, guanidine represents an important substructure in many biological molecules such as guanine , arginine or guanosine .
history
Guanidine was first synthesized in 1861 by the oxidative degradation of guanine. X-ray structural data of adducts of guanidine were obtained in 2007, but despite the simple molecular structure, the guanidine crystal structure was only fully elucidated 148 years after the first synthesis. Finally, in 2013 the position of the hydrogen atoms was determined very precisely using neutron diffraction on the guanidine single crystal.
Occurrence
Many natural substances are guanidine derivatives, including such important ones as the proteinogenic amino acid arginine , creatine and creatinine . The guanidine derivatives arginine and argininosuccinate play an important role in the urea cycle and thus in the detoxification of the ammonia formed by metabolic processes .
Chemical properties
Guanidine is an extremely strong base in water with a pK B value of 0.30 and is thus comparable to an alkali hydroxide . Before the discovery of proton sponges , guanidine was considered to be the strongest organic base. Therefore, on contact with moisture, z. B. with humidity, the guanidinium cation [C (NH 2 ) 3 ] + . The reactivity is sufficient to bind carbon dioxide from the air in the guanidinium carbonate. The basicity in the gas phase, however, was predicted to be significantly lower.
This high reactivity of guanidine and the very high stability of the guanidinium cation was tried to explain with different concepts. A simple suggestion was the greater mesomeric stabilization of the guanidinium ion compared to the free base. The concept of Y aromaticity is based on this; the guanidinium ion is not cyclic, but has six π electrons that are delocalized over the molecule . This should lead to stabilization. Other work, however, saw the predominant stabilization in the numerous hydrogen bonds between guanidinium and water molecules.
Guanidine can be understood as structurally related to carbonic acid, whereby the hydroxyl groups have been replaced by amino groups and the carbonyl group by an imino group : The formal, successive exchange of the oxygen functions in the carbonic acid structure by corresponding nitrogen-containing groups results in carbamic acid, then urea and finally guanidine.
Extraction and presentation
Adolph Strecker synthesized guanidine from guanidinium sulfate, which he obtained from guanine through oxidative degradation. To do this, he mixed the salt of sulfuric acid with barite water (a barium hydroxide solution) and evaporated the solvent in a vacuum. However, due to the high hygroscopicity of guanidine, he was unable to examine the free base.
Another synthetic protocol used a metathesis reaction to obtain guanidine. For this, potassium hydroxide was reacted stoichiometrically with guanidinium perchlorate in ethanol. The guanidinium hydroxide formed was dried over phosphorus pentoxide in vacuo in order to split off water.
In order to crystallize guanidine, guanidinium chloride was dissolved in THF and a sodium methoxide solution, likewise in THF, was added with exclusion of air. Acetonitrile was allowed to slowly diffuse into the solution . Single crystals of the adduct of guanidine and 3-amino-5,6-dimethyl- [1,2,4] triazine were formed, the latter as a result of a reaction of acetonitrile with guanidine in the presence of the alcoholate. For example, guanidine as a molecule could be examined by X-ray for the first time.
The pure, free base guanidine could be synthesized with a similar metathesis reaction. For this purpose guanidinium chloride was reacted with sodium ethoxide in ethanol - under a protective gas atmosphere - whereby sodium chloride precipitated (see reaction equation). The solution was filtered and the solvent was allowed to slowly evaporate. Single crystals precipitated out on cooling.
A single crystal large enough for neutron diffraction was grown by allowing a guanidine solution in ethanol to crystallize out for about half a year.
Derivatives
- Guanidine forms guanidinium salts with acids , e.g. B. guanidinium chloride , guanidinium thiocyanate , guanidinium nitrate and guanidinium carbonate
- Guanidine derivatives are used in the manufacture of flame retardants and resins . Coconut propylene diamine guanidinium acetate for disinfectants .
- A number of explosives are derived from guanidine, e.g. B .:
- Guanidinium nitrate
- Nitroguanidine
- Aminonitroguanidine
- Amino, diamino, triaminoguanidines and their salts
- Dinitroguanidine and its salts
- Tetrazene
- As medicaments, the biguanide is metformin in the treatment of type 2 diabetes using
- A number of derivatives of guanidine have an extremely sweet taste, up to 200,000 times the sweetness of sucrose . This makes them one of the sweetest compounds known to date.
Individual evidence
- ↑ a b c Entry on guanidine. In: Römpp Online . Georg Thieme Verlag, accessed December 10, 2014.
- ↑ a b Registration dossier on guanidines ( GHS section ) at the European Chemicals Agency (ECHA), accessed on March 13, 2019.
- ↑ Entry on guanidine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ^ Edward C. Franklin: The Ammono Carbonic Acids . In: Journal of the American Chemical Society . 44, 1922, pp. 486-509. doi : 10.1021 / ja01424a007 .
- ^ SJ Angyal, WK Warburton: The basic strengths of methylated guanidines . In: Journal of the Chemical Society (Resumed) . 1951, pp. 2492-2494. doi : 10.1039 / jr9510002492 .
- ↑ a b c d e A. Strecker, Liebigs Ann. Chem. 1861 , 118 , 151.
- ↑ a b c d T. Yamada, X. Liu, U. Englert, H. Yamane, R. Dronskowski: Solid-State Structure of Free Base Guaninide Achieved at Last . In: Chem Eur J.. . 15, 2009, p. 5651. doi : 10.1002 / chem.200900508 .
- ↑ a b R. Caminiti, A. Pieretti, L. Bencivenni, F. Ramondo, N. Sanna: Amidine N − C (N) −N Skeleton: Its Structure in Isolated and Hydrogen-Bonded Guanidines from ab Initio Calculations . In: The Journal of Physical Chemistry . 100, 1996, pp. 10928-10935. doi : 10.1021 / jp960311p .
- ^ A b M. Goebel, TM Klapoetke : First structural characterization of guanidine . In: Chem. Commun. . 43, No. 30, 2007, pp. 3180-3182. doi : 10.1039 / B705100J .
- ↑ a b P. K. Sawinski, M. Meven, U. Englert, R. Dronskowski: Single-Crystal Neutron Diffraction Study on Guanidine, CN 3 H 5 . In: Cryst Growth Des . 13, 2013, pp. 1730-1735. doi : 10.1021 / cg400054k .
- ↑ HR Christen, F. Vögtle: Organic chemistry - From the basics to research . 2nd edition, p. 425, Otto Salle Verlag, Frankfurt a. Main 1996, ISBN 3-7935-5398-1 .
- ^ A b Alberto Gobbi, Gernot Frenking: Y-Conjugated compounds: the equilibrium geometries and electronic structures of guanidine, guanidinium cation, urea, and 1,1-diaminoethylene . In: Journal of the American Chemical Society . 115, 1993, pp. 2362-2372. doi : 10.1021 / ja00059a035 .
- ^ A b Kenneth B. Wiberg: Resonance interactions in acyclic systems. 2. Y-conjugated anions and cations . In: Journal of the American Chemical Society . 112, 1990, pp. 4177-4182. doi : 10.1021 / ja00167a011 .
- ^ W. Marckwald, F. Struwe: About some guanidonium salts . In: Ber. . 55, 1922, pp. 457-463. doi : 10.1002 / cber.19220550221 .
- ↑ W. Jeremy Jones: The infra-red spectrum and structure of guanidine . In: Trans. Faraday Soc. . 55, 1959, pp. 524-531. doi : 10.1039 / TF9595500524 .
- ↑ H.-D. Belitz et al .: Textbook of Food Chemistry . 5th ed., Springer, Berlin et al. 2001. p. 433.