Guanidine hydrochloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
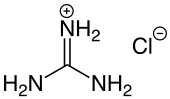
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Guanidine hydrochloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 5 N 3 • HCl | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 95.53 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.354 g cm −3 |
|||||||||||||||
| Melting point |
188 ° C |
|||||||||||||||
| Vapor pressure |
> 310 ° C (decomposition) |
|||||||||||||||
| solubility |
good in water (2150 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Guanidine hydrochloride , more guanidinium chloride is the chloride - salt of the guanidine - cation .
presentation
Guanidine hydrochloride is formed when cyanamide is reacted with ammonium chloride in an alcoholic solution at 100 ° C. The reaction equation is as follows:
use
It is used as a chaotropic renaturant or denaturant for proteins in biochemistry . It is also used as an additive for flux for soldering .
Individual evidence
- ↑ Data sheet guanidine hydrochloride (PDF) from Merck , accessed on December 14, 2010.
- ↑ a b c d e f g Entry on guanidinium chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on guanidinium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet guanidine hydrochloride (PDF) from Carl Roth , accessed on December 14, 2010.
- ^ LF Fieser, M. Fieser, in: Textbook of organic chemistry , 3rd edition, Verlag Chemie, 1957, page 267
- ↑ Werner A. Eckert, Jürgen Kartenbeck: Proteins: Standard methods of molecular and cell biology: preparation, gel electrophoresis, membrane transfer and immunodetection. Springer, 1997, ISBN 978-3-540-61278-0 , p. 32.
- ↑ David Nelson, Michael Cox, Albert L. Lehninger: Lehninger Biochemie. 3rd edition, Springer, 2005, ISBN 978-3-540-41813-9 , p. 200.
- ↑ Solder flux . Patent DE69420752T2 from May 18, 2000.


