Nitroguanidine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nitroguanidine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 4 N 4 O 2 | |||||||||||||||
| Brief description |
colorless, crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.06 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.77 g cm −3 |
|||||||||||||||
| Melting point |
239 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−98.74 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nitroguanidine (also abbreviated to NiGu or NQ ) is an energy-rich, chemical compound from the group of nitroimines, which is important as a component of propellant powder and safety explosives .
Nitroguanidine is an extremely insensitive explosive that, despite its low detonation energy, achieves a high detonation speed (8344 m / s) and a high detonation pressure of 29 GPa at a density of 1.742 g / cm 3, which comes close to the performance data of Hexogen .
Extraction and presentation
Nitroguanidine is produced by the action of cold concentrated sulfuric acid on guanidinium nitrate . It can also be produced by reacting dicyandiamide with ammonium nitrate or by reacting urea with ammonium nitrate.
properties
Nitroguanidine forms colorless, orthorhombic , needle-shaped crystals . Its density determined by X-ray is 1.77 g / cm³, its melting point is 239 ° C (sub., Decomp.). Nitroguanidine is not hygroscopic . It is sparingly soluble in cold water, methanol and ethanol , soluble in hot water (slow hydrolysis ), acids and bases (decomposition). It forms addition compounds with ketones and alcohols .
Nitroguanidine forms two crystal habitus α-nitroguanidine and β-nitroguanidine, which are diffractometrically identical. Both forms are not changed by recrystallization from water, glacial acetic acid or ammonia . If β-nitroguanidine is dissolved in 96% sulfuric acid and the solution is introduced into water, α-nitroguanidine separates out.
The thermal decomposition of the compound becomes relevant at temperatures above 150 ° C. As decomposition products, nitrous oxide , ammonia , nitrogen dioxide , carbon monoxide and carbon dioxide are observed.
Explosion parameters
Nitroguanidine is very insensitive and only detonates after initiation with an ignition amplifier . Important explosion indicators are:
- Heat of explosion 3062 kJ kg −1 .
- Detonation temperature 2800 K at maximum density calculated from
- Detonation velocity : 8546 m · s −1 at the maximum density
- Detonation pressure : 29 GPa
- Detonation temperature : 2811 K
- Impact sensitivity up to 50 Nm no reaction
- Friction sensitivity up to 353 N pin load no reaction
- Critical diameter at a density of 1.52 g / cm 3 <14 mm
- Steel sleeve test with a limit diameter of 1 mm no ignition.
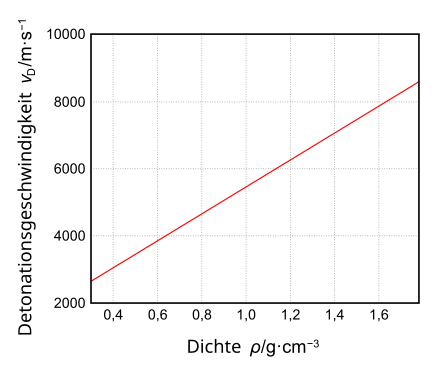
As with all explosives, the detonation velocity, v D , of nitroguanidine increases with its density. v D follows the following law in the range from 0.3 to 1.78 g · cm −3 : v D = 1.44 + 4.015 · density [mm · µs −1 ] (see also the following graphic)
Nitroguanidine is one of the strong but difficult to detonate explosives. This explains the strong dependence of the detonation speed on the diameter. A charge with a density of 0.95 g / cm 3 has a detonation speed of 4340 m / s in a pipe with an internal diameter of 20 mm.
use
Nitroguanidine is used in gas-generating pyrotechnic sets for airbags and in so-called "cold" three-base propellant charge powders, which are gentle on the barrel and produce less muzzle flash. NiGu is used as an extremely insensitive but powerful explosive in explosives such as AFX-760, IMX-101 and AlIMX-101 are used.
Nitroguanidine comes on the market as fine-needle LBDNQ (low bulk density nitroguanidine) as well as granular HBDNQ (high bulk density NQ) and very rarely as spherical SHBDNQ (spherical high bulk density NQ).
Nitroguanidine is a building block for insecticides from the fastest growing class of neonicotinoids , the most important representatives of which are imidacloprid (Bayer Crop Science), clothianidin (Takeda, Bayer Crop Science), thiamethoxam (Syngenta) and dinotefuran ( Mitsui Chemicals ).
structure
Many sources give an incorrect structural formula for nitroguanidine, according to which NQ would be a nitramine. However, neutron diffraction and 1 H and 15 N NMR experiments clearly confirm that nitroguanidine is a nitroimine.
Individual evidence
- ↑ a b c Entry on 1-nitroguanidine in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c d e f g Ernst-Christian Koch: Insensitive High Explosives: III. Nitroguanidine - Synthesis - Structure - Spectroscopy - Sensitiveness . In: Propellants, Explosives, Pyrotechnics . tape 44 , no. 3 , March 2019, p. 267–292 , doi : 10.1002 / prep.201800253 .
- ↑ Data sheet Nitroguanidine from Sigma-Aldrich , accessed on January 27, 2013 ( PDF ).
- ↑ a b c d e f E.-C. Koch: explosives, propellants, pyrotechnics. 2nd, completely revised edition. DeGruyter, Berlin, 2019, ISBN 978-3-11-055784-8 .
- ^ A b c Ernst-Christian Koch: Insensitive high explosives: IV. Nitroguanidine - Initiation & detonation . In: Defense Technology . tape 15 , no. 4 , August 2019, p. 467-487 , doi : 10.1016 / j.dt.2019.05.009 .
- ↑ a b Yanchun Li, Yi Cheng: Investigation on the thermal stability of nitroguanidine by TG / DSC-MS-FTIR and multivariate non-linear regression. In: J. Therm. Anal. Calorim. 100, 2010, pp. 949-953 ( doi: 10.1007 / s10973-009-0666-3 ).
- ^ A b R. Doherty, RL Simpson: Comparative Evaluation of several insensitive high explosives, 28th International Annual ICT Conference, June 1997, Karlsruhe, Germany. V-32.
- ↑ a b Terry R. Gibbs, Alphonse Popolato: LASL explosive property data . University of California Press, 1984, ISBN 0-520-04012-0 , pp. 52–60 ( limited preview in Google Book Search).
- ↑ P. Maienfisch: Synthesis and Properties of Thiamethoxam and Related Compounds. In: Journal of Nature Research B . 61, 2006, pp. 353–359 ( PDF , free full text).
- ↑ J. Köhler, R. Meyer, A. Homburg, Explosivstoffe , 10th edition, Wiley-VCH, Weinheim, 2008 , pp. 216-217
- ↑ CS Choi: Refinement of 2-Nitroguanidine by Neutron Powder Diffraction. In: Acta Cryst. B . 37, 1981, pp. 1955-1957. doi: 10.1107 / S0567740881007735 .
- ↑ S. Bulusu, RL Dudley, JR Autera: Structure of Nitroguanidine: Nitroamine or Nitroimine? New NMR Evidence from 15 N-Labeled Sample and 15 N Spin Coupling Constants. In: Magnetic Resonance in Chemistry . 25, 1987, pp. 234-238. doi: 10.1002 / mrc.1260250311 .