Imidacloprid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Imidacloprid | |||||||||||||||||||||
| other names |
1- (6-chloro-3-pyridinylmethyl) - N -nitroimidazolidin-2-ylideneamine |
|||||||||||||||||||||
| Molecular formula | C 9 H 10 ClN 5 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 255.66 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.54 g cm −3 |
|||||||||||||||||||||
| Melting point |
136.4 or 143.8 ° C (two crystal forms) |
|||||||||||||||||||||
| Vapor pressure |
0.2 µ Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Imidacloprid is a systemic insecticide from the group of neonicotinoids . The substance was first synthesized in 1985 in the laboratories of Bayer AG . Bayer has been manufacturing imidacloprid on an industrial scale since the early 1990s; it is used in around 120 countries around the world. Some experts believe that imidacloprid is currently the most widely used insecticide in the world.
Extraction and presentation
Imidacloprid can be obtained from 2-chloro-5-formylpyridine through a multi-stage reaction with ethylenediamine , sodium borohydride , cyanogen bromide and nitric acid.
Alternative synthesis variants are also known, for example by reacting ethylenediamine with cyanogen bromide and nitric acid to form 2-nitroimine-imidazolidine and reacting it with 2-chloro-5-chloromethylpyridine.
economy
The annual sales in Germany are in the range of 25-100 t, over 1000 t are exported. Sales are around 500–600 million euros, making Imidacloprid the most successful product from Bayer CropScience . Trade names for the insecticide are Admire , Confidor (in Europe), Connect , Evidence , Gaucho (stain), Leverage , Muralla , Provado and Trimax .
Mode of action
Imidacloprid is a systemic insecticide that can act as a contact poison as well as a food poison. It is well absorbed by the roots and transported into the leaves, which are then protected from biting and sucking insects. If it is applied directly to the leaves, it is distributed between the top and bottom of the leaf and is also transported on to the newly formed leaves. Since imidacloprid is only slowly broken down in the plant, its effects last for a long time.
In insects, imidacloprid acts like acetylcholine on the nicotinic acetylcholine receptor of the nerve cells , but it is not broken down by the enzyme acetylcholinesterase . The chemical signal transmission is disturbed by the triggered permanent stimulus.
use
Plant protection
Numerous pesticides containing imidacloprid are approved in Germany, Austria and Switzerland as well as in other EU countries. They come in the form of suspensions , water-soluble concentrates, granules , sticks (for potted plants), powdered slurry (for seed treatment) or spray . Imidacloprid is often the only active ingredient, there are also combination products with the pyrethroid tefluthrin , the carbamate methiocarb , other insecticidal active ingredients and with nutrients .
Imidacloprid can be used for the seed dressing of sugar beet and fodder beet , grain, potatoes , corn , onions and the oil pumpkin . Here it works, for example, against plant lice , wireworms , Colorado beetles as well as the fried , onion and beet flies . Imidacloprid is approved for seed treatment of rapeseed (against the rapeseed flea ) in Germany and Austria, but not in Switzerland.
In house u. In allotments, Imidacloprid is used against plant lice (including whitefly ) and thrips .
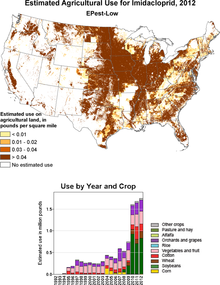
In 2012, around 750 t of imidacloprid were used in the USA, almost half of which were used in soy cultivation.
Veterinary medicine
Imidacloprid has been used against animal lice and fleas in dogs and cats since 1996 . Trade names are Advantage , Clearspot (marketable until January 23, 2017) and Midaspot . It is also contained in combination preparations with permethrin ( Advantix and Ataxxa ), with moxidectin ( Advocate and Prinovox ) and with flumethrin ( Seresto ).
Biocidal products
According to Directive 98/8 / EC of February 16, 1998, only those biocidal products should be permitted whose active substances have been included in Annexes I, IA and IB of the said directive (for the respective defined product type). In accordance with the transitional regulation (Art. 16 (1) of Directive 98/8 / EC), the placing on the market of biocidal products with so-called "Old active substances", ie those that are not listed in the annexes of Directive 98/8 / EC, but were already on the market with the reference date May 14, 2000.
According to Regulation (EC) 1896/2000 of September 7, 2000, manufacturers who wanted to apply for the inclusion of an “old active substance” in Annexes I, IA and IB had to notify the relevant active substance for the corresponding product type by March 28, 2002 have reported. This period was extended by Regulation (EC) 1687/2002 from September 25, 2002 to January 31, 2003. The “notified active substances” were allowed to remain on the market until the final decision on inclusion or non-inclusion in Annex I, IA and IB of EU Directive 98/8 / EC.
The active ingredient imidacloprid was added to the list of notified active ingredients for product type 18 (insecticides).
With Regulation (EC) 1451/2007 of December 4, 2007 on the second phase of the ten-year work program on placing biocidal products on the market, the active substance imidacloprid was included in the final list (Annex II) of those to be examined as part of the test program Active ingredients added.
With the adoption of Directive 2011/69 / EU of July 1, 2011, a decision has been made to include the active ingredient imidacloprid in the corresponding list (Annex I of Directive 98/8 / EC) for product type 18 (insecticides) from July 1, 2013 . The sale of biocidal products containing the active ingredient imidacloprid was still permitted in the EU (Switzerland has adopted this provision) for product type 18 (insecticides).
With the enactment of the Implementing Regulation (EU) No. 783/2018 of May 24, 2018, the use of the neonicotinoids clothianidin, thiamethoxam and imidacloprid in outdoor crops was banned in the EU.
Toxicity and Ecotoxicity
Mammals
The acute toxicity of imidacloprid is low for mammals ; in male rats the LD 50 was found to be 424 mg / kg body weight. In three-month feeding tests , the NOEC (no observed effect concentration) was 150 mg / kg feed in male rats, 600 in female rats and 200 mg / kg feed in dogs. Imidacloprid is not irritating to the skin or eyes. The substance presumably has a weak teratogenic and mutagenic effect. It is not considered to be carcinogenic . Imidacloprid is quickly absorbed into the body through the gastrointestinal tract. About 80% of it is broken down in the body within 48 hours and about 20% is excreted unchanged.
Concentrations greater than 1 μ M imidacloprid triggered newborn rat nerve impulses in the brain cells. Imidacloprid may have effects on nervous system development in humans and animals similar to nicotine.
fishes
Imidacloprid is moderately toxic to aquatic organisms; the lethal concentration in the 96-hour test for rainbow trout is 211 mg / L. In the endurance test with rainbow trout over 21 days, no effect was observed below a concentration of 28.5 mg / L.
Imidacloprid exposure of zebrafish and medaka resulted in malformations , lesions and reduced growth .
Invertebrates
Water fleas ( Daphnia magna ) are more sensitive, after 48 hours 85 mg / L caused half of the daphnia to stop their rowing movements. Over a period of 21 days, there were no more effects on daphnia at concentrations below 1.8 mg / L. Imidacloprid, on the other hand, is highly toxic to aquatic insects. The acutely toxic concentrations for z. B. stone and mayfly larvae in the range of a few µg / L.
Birds
Imidacloprid is poisonous for birds , the lethal dose for canaries and pigeons is in the range of 25–50 mg / kg body weight. When birds peck dressed seeds from the fields, they are at risk of acute poisoning.
A large number of golden siskins died after using imidacloprid on street trees in Modesto , California . They had eaten contaminated tree seeds. The imidacloprid concentration in the contents of the goiter and stomach averaged 4.8 mg / kg, the liver tissue contained 3.9 mg / kg.
In the Netherlands, the concentration of imidacloprid in surface water showed a statistical association with the decline in several insectivorous bird species since the mid-1990s. Regarding a possible causal connection, the authors speculate that the use of imidacloprid has decimated the food sources of these bird species.
Bees
A meta-analysis of 14 studies published in 2011 regarding the effects of imidacloprid on honey bees under laboratory and semi-field conditions showed that the dosages expected under field conditions would not have lethal effects, but would reduce the bees' performance by six to twenty percent .
A systematic review from 2012 found that many laboratory studies had shown lethal and sub- lethal effects of neonicotinoids on the ability to procure food, learn and remember, while studies under realistic field conditions with correspondingly lower doses had shown no effects.
A review article also published in 2012 could not support the hypothesis of a colony collapse due to neonicotinoid residues in pollen and nectar based on the Bradford Hill criteria for the time being, as there are considerable gaps in knowledge.
According to a review published in 2014, a single causal relationship between the use of neonicotinoids and bee deaths cannot yet be concluded due to gaps in knowledge. The bee mortality occurred before the widespread use of neonicotinoids and there was a weak geographical correlation between neonicotinoid use and bee deaths.
A review article on neonicotinoids, also published in 2014, compared a number of recent laboratory studies with field studies. While laboratory studies have found sublethal effects, these effects have not been proven in field studies. The authors conclude that the laboratory studies overestimated the bees' concentration, feeding time and food choices.
A systematic review published in August 2015 (Lundin et al., 2015) examined the research methods and gaps in research on neonicotinoids and bees using 216 individual studies published by June 2015. The authors concluded that despite numerous research activities, there are still significant knowledge gaps. Most of the studies looked at Europe and North America and a few crops (corn, oilseed rape, sunflower) and species (mainly Apis mellifera ), although the relationships in other regions, crops and species may be different. In addition, despite many laboratory studies, there is a lack of field studies, and field studies have primarily examined the exposure of bees to neonicotinoids, but there is insufficient knowledge about the effects of this exposure. Furthermore, research so far has focused on individual bees, although the effects on bee colonies can be different. Although there are indications of interactions between different classes of insecticide and synergistic insecticide-pathogen / parasite interactions, the latter may have been overestimated under realistic field conditions. Research also needs to clarify how relevant neonicotinoids are compared to other possible causes of bee mortality.
Environmental behavior
The active ingredient is only slowly broken down in the environment. The rate of degradation depends on the intensity of life in the soil . In an examination half of the imidacloprid applied had decomposed within 48 days on overgrown soil; on ungrown soil this state was only reached after 190 days. As metabolite is 6-chloronicotinic acid formed.
regulation
EU
The use of imidacloprid is approved in the EU and 25 member states, but due to risks for honey bees, from December 1, 2013, for two years initially for several important uses, such as the seed dressing of maize and rapeseed, severely restricted (see Neonicotinoids # EU- Restrictions from 2013 ). The permitted daily dose is 0.06, the acute reference dose 0.08 and the acceptable user exposure 0.08 milligrams per kilogram of body weight and day.
The European Food Safety Authority proposed in December 2013 that the Acute Reference Dose and Acceptable User Exposure should be reduced from 0.08 to 0.06 mg / kg body weight due to possible effects on the developing nervous system. The permitted daily dose was considered to be adequate to ensure protection against possible developmental neurotoxic effects.
Most of the approvals for dressings containing imidacloprid apply to sugar beet, maize, potatoes, rape and cereals, as well as a number of other plant species.
An EFSA opinion published on February 28, 2018 finally confirmed the risks for wild and honey bees in field applications. This report is intended to serve as a basis for further approval decisions or restrictions.
On April 27, 2018, the EU Commission passed a vote to ban outdoor crops.
In August 2018, the approval for outdoor use was revoked on September 18, 2018. Pesticides with this active ingredient may only be used in permanent greenhouses and for the treatment of seeds that are intended for application in the greenhouse. Treated seeds, which are intended for sowing in the open, may be sown until December 18, 2018.
literature
- Caroline Cox: Insecticide Factsheet Imidacloprid . Journal of Pesticide Reform , Spring 2001, Vol. 21, No. 1.
Web links
- EXTOXNET: Pesticide Information Profile Imidacloprid (engl.)
- Entry on Imidacloprid at Vetpharm
Individual evidence
- ↑ a b c d e f EXTOXNET: Pesticide Information Profile Imidacloprid .
- ↑ a b Entry on Imidacloprid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on imidacloprid (ISO); 1- (6-chloropyridin-3-ylmethyl) -N-nitroimidazolidin-2-ylidenamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 11, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Imidacloprid data sheet from Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ a b Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 448 ( limited preview in Google Book search).
- ↑ a b Directorate-General for Health and Food Safety of the European Commission: Entry on Imidacloprid in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 16, 2016.
- ↑ Rapeseed treated with imidacloprid. Use harmful to health. Retrieved March 19, 2019 .
- ↑ EU: Directive 98/8 / EC of February 16, 1998 on the placing of biocidal products on the market (PDF) Official Journal of the European Communities L 123/1 of April 24, 1998
- ↑ EU: Regulation (EG) 1896/2000 of 7 September 2000 on the first phase of the program in accordance with Article 16, Paragraph 2 of Directive 98/8 / EC on biocidal products (PDF) Official Journal of the European Communities L 228/6 of September 8, 2000
- ↑ EU: Regulation (EG) 1687/2002 of September 25, 2002 on an additional deadline for the notification of certain active substances (PDF) Official Journal of the European Communities L 258/15 of September 26, 2002
- ↑ EU: Regulation (EG) 2032/2003 of November 4, 2003 on the second phase of the ten-year work program on placing biocidal products on the market (PDF) Official Journal of the European Communities L 307/1 of November 24, 2003
- ↑ EU: Regulation (EG) 1451/2007 on the second phase of the ten-year work program on placing biocidal products on the market (PDF) Official Journal of the European Communities L 325/3 of December 11, 2007
- ↑ EU: Directive 2011/69 / EU of July 1, 2011 amending Directive 98/8 / EC to include imidacloprid in Appendix I (PDF) Official Journal of the European Communities L 243/16 of July 1, 2011
- ↑ EU: Commission Regulation (EU) 2018/783 of May 29, 2018 amending Implementing Regulation (EU) No. 540/2011 with regard to the conditions for the approval of the active substance imidacloprid
- ↑ Junko Kimura-Kuroda, Yukari Komuta, Yoichiro Kuroda, Masaharu Hayashi, Hitoshi Kawano: Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats . In: PLoS ONE . tape 7 , no. 2 , 2012, doi : 10.1371 / journal.pone.0032432 .
- ↑ Stephanie Schnydrig: Neurotoxins damage aquatic organisms differently than expected. In: eawag.ch . June 13, 2019, accessed July 17, 2019 .
- ↑ Caroline Vignet, Tiziana Cappello, Qiuguo Fu, Kévin Lajoie, Giuseppe De Marco, Christelle Clérandeau, Hélène Mottaz, Maria Maisano, Juliane Hollender, Kristin Schirmer, Jérôme Cachot: Imidacloprid induces adverse effects on fish early life stages that are more severe in Japanese medaka (Oryzias latipes) than in zebrafish (Danio rerio). In: Chemosphere. 225, 2019, p. 470, doi : 10.1016 / j.chemosphere.2019.03.002 .
- ↑ Francisco Sánchez-Bayo, Koichi Goka, Daisuke Hayasaka: Contamination of the Aquatic Environment with Neonicotinoids and its Implication for Ecosystems . In: Frontiers in Environmental Science . tape 4 , 2016, ISSN 2296-665X , doi : 10.3389 / fenvs.2016.00071 ( frontiersin.org [accessed September 27, 2019]).
- ^ Krysta H. Rogers, Stella McMillin, Katie J. Olstad, Robert H. Poppenga: Imidacloprid poisoning of songbirds following a drench application of trees in a residential neighborhood in California, USA. In: Environmental Toxicology and Chemistry . 2019, doi: 10.1002 / etc.4473 .
- ↑ Caspar A. Hallmann, Ruud PB Foppen, Chris AM van Turnhout, Hans de Kroon, Eelke Jongejans: Declines in insectivorous birds are associated with high neonicotinoid concentrations . In: Nature . July 9, 2014, doi : 10.1038 / nature13531 .
- ↑ James E. Cresswell: A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees . In: Ecotoxicology . tape 20 , no. 1 , 2011, p. 149–157 , doi : 10.1007 / s10646-010-0566-0 .
- ^ Final report dressing and bee damage . Ministry of Food and Rural Areas Baden-Württemberg , December 17, 2008.
- ↑ T. Blacquière, G. Smagghe, CA. van Gestel, V. Mommaerts: Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment . In: Ecotoxicology . tape 21 , no. 4 , 2012, p. 973-992 , doi : 10.1007 / s10646-012-0863-x .
- ↑ James E Cresswell, Nicolas Desneux, Dennis van Engelsdorp: Dietary traces of neonicotinoid pesticides as a cause of population declines in honey bees: an evaluation by Hill's epidemiological criteria . In: Pest Management Science . tape 68 , no. 6 , 2012, p. 819-827 , doi : 10.1002 / ps.3290 .
- ↑ H. Charles J. Godfray, Tjeerd Blacquière, Linda M. Field, Rosemary S. Hails, Gillian Petrokofsky, Simon G. Potts, Nigel E. Raine, Adam J. Vanbergen, Angela R. McLean: A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators . In: Proceedings of the Royal Society B . tape 281 , no. 1786 , 2014, p. 20140558 , doi : 10.1098 / rspb.2014.0558 .
- ↑ Norman L Carreck, Francis LW Ratnieks: The dose makes the poison: have “field realistic” rates of exposure of bees to neonicotinoid insecticides been overestimated in laboratory studies? In: Journal of Apicultural Research . tape 53 , no. 5 , 2014, p. 607-614 , doi : 10.3896 / IBRA.1.53.5.08 .
- ↑ Ola Lundin, Maj Rundlöf, Henrik G. Smith, Ingemar Fries, Riccardo Bommarco: Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps . In: PLOS One . August 27, 2015, p. 1-20 , doi : 10.1371 / journal.pone.0136928 .
- ↑ EFSA: EFSA assesses possible association between two neonicotinoids and developmental neurotoxicity , press release of December 17, 2013.
- ↑ EFSA: Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid . In: EFSA Journal . tape 11 , no. 1 , 2013, p. 3068 , doi : 10.2903 / j.efsa.2013.3068 .
- ↑ Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU | European Food Safety Authority. Accessed March 19, 2018 (English).
- ↑ EFSA: Q&A: Conclusions on Neonicotinoids 2018 , February 28, 2018.
- ↑ Fight against bee mortality: EU bans neonicotinoids in fields ( memento of July 24, 2018 in the Internet Archive ) br.de , April 27, 2018.
- ↑ Federal Office for Consumer Protection and Food Safety: BVL - Fachmachrichten - Revocation of the approval of plant protection products with the neonicotinoid active ingredients Clothianidin, Imidacloprid and Thiamethoxam on September 18, 2018 , accessed on December 8, 2018.