Acetylcholine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
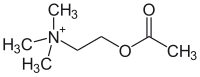
|
|||||||||||||
| Counterion (mostly chloride) not shown | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Acetylcholine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 7 H 16 NO 2 | ||||||||||||
| Brief description |
white, crystalline powder or colorless crystals, very hygroscopic (chloride) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
149–152 ° C (chloride) |
||||||||||||
| solubility |
very easily soluble in water, slightly soluble in ethanol , sparingly soluble in dichloromethane (chloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Acetylcholine ( ACh ) is one of the most important neurotransmitters in many organisms, including humans . The quaternary ammonium compound acetylcholine is an ester of acetic acid and the monohydric amino alcohol choline .
history
In 1921, Otto Loewi demonstrated using a frog's heart that a chemical substance is responsible for the transmission of a nerve impulse to the heart , which he initially called vagus substance and which Henry H. Dale later identified as acetylcholine.
The substance was first synthesized in 1867 by Adolf Baeyer , with whose substance the American Reid Hunt (1870–1948) was able to trigger muscle contractions in animal experiments.
Occurrence
Acetylcholine is found in both the central and peripheral nervous systems .
In the autonomic nervous system it is the transmitter of all preganglionic-autonomic neurons. In addition, it mediates the signal transmission of the postganglionic parasympathetic neurons to the end organs. The postganglionic sympathetic neurons normally use noradrenaline ; the pathways to the sweat glands are an exception in that they also use ACh as a transmitter.
In the peripheral nervous system, ACh mediates the transmission of excitation from the nerves to the muscles via the neuromuscular endplate .
In the central nervous system, ACh is found in the corpus striatum , in the nucleus basalis Meynert with channels to the cerebral cortex and in the formatio septalis medialis with channels to the hippocampus . After γ-aminobutyric acid (GABA) and glycine, acetylcholine is one of the most common neurotransmitters in the brain .
function
Cellular function
Acetylcholine acts on two types of cholinergic receptors , the nicotinic acetylcholine receptor and the muscarinic acetylcholine receptor . They got their name from substances that specifically activate them, nicotine for the nicotinic receptor and muscarinic for the muscarinic receptor. There are different subtypes of both.
The nicotinic ACh receptor is a ligand-controlled ion channel which, when opened, i.e. after ACh binding, is permeable to sodium, potassium and calcium ions. There are two subtypes of the receptor, a muscle type which can be selectively inhibited by curare and a neuron type which can be selectively inhibited by hexamethonium .
The muscarinic ACh receptor is a G protein-coupled receptor with 5 subtypes (M1-M5). M1, M3 and M5 are G q -coupled, M2 and M4 G i / G o -coupled.
There are also substances that can indirectly increase the effect of ACh on its receptors (indirectly cholinergic substances). These include the various inhibitors of cholinesterase (actually acetylcholinesterase inhibitors). These are very different inhibiting substances. The aforementioned drugs that are used in Alzheimer's disease belong to the large group of these substances. Some cause a temporary inhibition, others block the enzyme permanently. Such irreversibly acting inhibitors of cholinesterase are various organophosphoric acid esters , e.g. B. the well-known insecticide Parathion (E 605), but also the chemical warfare agents Sarin , Tabun , Novitschok and many others, which cause fatal overstimulation of the cholinergic synapses in small amounts. The substance neostigmine belongs to the group of reversible cholinesterase inhibitors . Numerous substances also block the action of acetylcholine on its receptors (especially on the muscarinic receptors); this is then called an anticholinergic effect. Certain alkaloids have an anticholinergic effect, for example atropine or hyoscyamine or scopolamine .
Cholinesterase inhibitors such as parathion or neostigmine are also used as an antidote for curare . Curare blocks the docking points for acetylcholine on the motor end plates and thereby paralyzes the skeletal muscles, which leads to death by suffocation. Since the active ingredients of Curare are competitive blockers, a lot of acetylcholine can displace them. If the acetylcholinesterase is blocked, more acetylcholine remains in the synaptic gap and the transmission works again. However, since the acetylcholine concentration also increases at the muscarinic receptors, follow-up treatment with atropine is often necessary. This effect is also used in general anesthesia: Before the operation, the muscles are paralyzed with a neuromuscular blocker (e.g. rocuronium ). A reversible cholinesterase inhibitor (e.g. neostigmine) is administered to reverse its effect. In addition, a parasympatholytic (e.g. atropine) is used to reverse the muscarinic side effects.
Physostigmine (eserin) is also a cholinesterase inhibitor and prevents the splitting of acetylcholine into choline and acetate by the cholinesterase.
Function in the central nervous system
In the central nervous system, ACh plays an important role in increasing waking alertness, maintaining alertness, learning, and creating memories.
Biosynthesis and metabolism
Acetylcholine is formed from acetyl-CoA and choline by the enzyme choline acetyltransferase .
The finished ACh is taken up from the cytosol via a proton / acetylcholine antiporter in the vesicle membrane into the neurosecretory storage vesicles . Each vesicle contains 5,000 to 10,000 acetylcholine molecules. There are approximately one million storage vesicles per synapse.
The enzyme acetylcholinesterase , after it has been released into the synaptic gap and bound to the acetylcholine receptor , can be broken down again into choline and acetic acid (or acetate ) and rendered ineffective.
Provision of the starting molecules for acetylcholine formation
Choline can be taken up again into the synapse from the synaptic gap via a sodium-choline symporter and is a breakdown product of the acetylcholine released last. The availability and re-uptake of choline represents the rate-limiting step for ACh synthesis because it cannot be produced by the nerve cell itself.
Pyruvate is the end product of glycolysis in the cytosol of every cell. The enzyme pyruvate dehydrogenase catalyzes the conversion of pyruvate to acetyl coenzyme A. Since acetyl-CoA, the inner mitochondrial membrane can not happen, it is in the citric acid cycle fed and by reaction with oxaloacetate (catalyzed by citrate synthase ) in citrate converted. The citrate crosses the mitochondrial membrane and is broken down again into acetyl-CoA and oxaloacetate by the citrate lyase . Acetyl-CoA is therefore available for the formation of acetylcholine.
Diseases involving the cholinergic system
In Alzheimer's disease , the death of nerve cells that mainly produce acetylcholine causes a deficiency in acetylcholine. One tries to compensate for this deficiency with medication by using acetylcholinesterase inhibitors to inhibit this acetylcholine-degrading enzyme in order to increase the acetylcholine concentration at the synapses . Another way is through the administration of precursor proteins such. B. Deanol and Meclophenoxat to increase the level of awareness.
The increased accumulation of acetylcholine due to a reduced breakdown of the substance causes a cholinergic crisis .
In myasthenia gravis muscle weakness , a serious autoimmune disease, antibodies are produced that destroy the acetylcholine receptors in muscle cells and thus weaken muscle activity. This can go so far that patients can no longer keep their eyes open.
Acetylcholine in animal and plant poisons
The proportion of acetylcholine in the poison of the hornet ( Vespa crabro ) is around six percent of the dry weight and is therefore in the highest concentration that has been found in a living being. The sting of the hornet is perceived as particularly painful due to this high concentration. The sting is no more toxic than other wasps or bees ( bee venom ), whose venom does not contain acetylcholine. Acetylcholine is also responsible for the painful effect in the poison of nettles , lionfish and other scorpion fish .
use
In ophthalmology , acetylcholine is used to constrict and reposition the preoperatively enlarged pupil after extraction of the lens in cataract operations , iridectomy , perforating keratoplasty and other interventions on the anterior segment of the eye, if this requires very quick and complete miosis .
According to the EC Cosmetics Directive, acetylcholine must not be contained in cosmetic products .
See also
Individual evidence
- ↑ a b c Datasheet Acetylcholine Chloride CRS (PDF) at EDQM , accessed on January 24, 2009.
- ↑ Acetylcholine data sheet from Acros, accessed on February 22, 2010.
- ↑ a b Data sheet Acetylcholine chloride from Sigma-Aldrich , accessed on March 2, 2019 ( PDF ).
- ^ Otto Westphal , Theodor Wieland , Heinrich Huebschmann: life regulator. Of hormones, vitamins, ferments and other active ingredients. Societäts-Verlag, Frankfurt am Main 1941 (= Frankfurter Bücher. Research and Life. Volume 1), p. 33 f. and 81 f.
- ↑ Barbara E. Jones: From waking to sleeping: neuronal and chemical substrates . In: Trends in Pharmacological Sciences . tape 26 , no. 11 , November 2005, p. 578-586 , doi : 10.1016 / j.tips.2005.09.009 , PMID 16183137 .
- ↑ AM Himmelhub, M. Sarter, JP Bruno: Increases in cortical acetylcholine release during sustained attention performance in rats . In: Brain Research. Cognitive Brain Research . tape 9 , no. 3 , 2000, pp. 313-325 , PMID 10808142 .
- ↑ RM Ridley, PM Bowes, HF Baker, TJ Crow: An involvement of acetylcholine in object discrimination learning and memory in the marmoset . In: Neuropsychologia . tape 22 , no. 3 , 1984, pp. 253-263 , PMID 6431311 .
- ^ PT Francis, AM Palmer, M. Snape, GK Wilcock: The cholinergic hypothesis of Alzheimer's disease: a review of progress . In: Journal of Neurology, Neurosurgery, and Psychiatry . tape 66 , no. 2 , 1999, p. 137-147 , PMID 10071091 , PMC 1736202 (free full text).
- ↑ Eric Kandel: In search of memory, 2009 3rd edition, Goldmann, Munich
