Nicotinic acetylcholine receptor
| Nicotinic acetylcholine receptor | ||
|---|---|---|
|
||
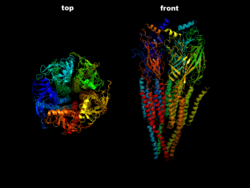
| The ACh-n receptor from the electric ray ( torpedo ) in side view (right) and top view (left) according to PDB 2BG9 | ||
| Mass / length primary structure | 2392 = 2 * 462 + 478 + 517 + 473 amino acids (2α 1 β 1 γε) | |
| Secondary to quaternary structure | Pentamer ααβδγ or ααβδε | |
| Transporter classification | ||
| TCDB | 1.A.9.1.1 | |
| designation | Ligand-gated ion channels | |
| Occurrence | ||
| Parent taxon | Multicellular animals | |
Nicotinic acetylcholine receptors ( nAChR ), also known as nicotine receptors for short , are membrane-based receptors in various nerve cells and muscle fibers that bind the neurotransmitter acetylcholine (ACh) as a substrate , but can also be activated by nicotine and similar nicotinergic substances. These ionotropic receptors consist of five subunits which, in addition to the receptive binding sites, together form a pore spanning the cell membrane and thus represent a ligand-controlled ion channel . This is a non-specific cation channel, which is especially permeable to monovalent Na + and K + ions. In contrast, the muscarinic acetylcholine receptors are metabotropic membrane-bound receptors.
The nicotinic receptors on the neuromuscular endplate , where impulses from nerve cells are synaptically transmitted to muscle fibers , have been well studied. To open the ion channel, two ACh molecules must bind to a receptor, which is more likely to happen with high transmitter concentrations than with low ones. In the case of myasthenia gravis , these ACh receptors are destroyed by autoimmune processes and the transmission of neuronal signals to the muscle is disrupted.
construction
This receptor is considered to be the prototype of its class. It is related to the ionotropic serotonin (5-HT 3 ) receptor , the GABA A receptor and the glycine receptor. The nicotinergic ACh receptor is a cylindrical transmembrane protein consisting of 5 subunits (2x α1-, β1-, γ-, δ-subunit), each of which spans the cell membrane four times (γ is replaced by ε in adult muscles). DNA sequencing revealed that the two α subunits in turn are composed of eight sub-types: α 1 -α 8 . The occurrence of different subunits, each of which can form a receptor complex, creates a multitude of different receptor isoforms, which are characterized by a different composition of the subunits, have different pharmacokinetic properties and are specifically distributed between organs and within the CNS . One can roughly differentiate between a muscle type N M and a neuron type N N of the N-cholinoceptors, depending on whether the nicotinic receptor is located in the muscles or the nervous system.
kinetics
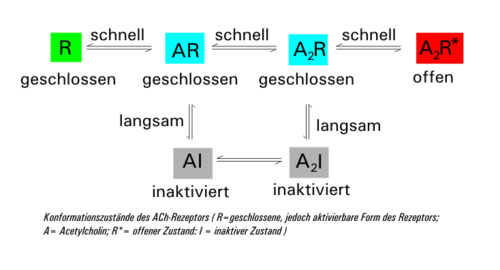
The α-subunits of the receptor each have a binding site for acetylcholine (two binding sites per receptor). When these binding sites are occupied by the transmitter, the interaction with the bound molecule changes the three-dimensional structure and a channel pore opens through which ions can pass through the cell membrane. This mechanism was only discovered recently. Sodium and calcium ions therefore flow into the cell, potassium flows out of the cell (in significantly smaller quantities). The shifts in charge create a current across the cell membrane, which, depending on its direction (from the outside into the cell), is called the depolarization of the cell, as it reduces the pre-existing polar charge distribution on the cell membrane (inside negative, outside positive) and even briefly reverses it can.
The ligand-occupied receptor comes in two forms: once as a closed (A 2 R) and once as an open (A 2 R *). These two states can quickly merge. At the same time, the closed form (A 2 R) changes with a time delay into the inactive form (A 2 I) (desensitization), which is converted back into the closed form AR with further elimination of ACh via AI. If the acetylcholine concentration falls below 10 nM again due to the activity of acetylcholinesterase , the receptor regenerates to its original form R. The time constant of desensitization (mean time to close the channel) is around 20-50 at an ACh concentration of 1 mM ms.
Due to Brownian molecular motion , the ACh molecules diffuse again after a short time from the binding site and are broken down by the enzyme acetylcholinesterase to choline and acetate or acetic acid. This closes the receptor again.
Pharmacological influence
Numerous plant alkaloids , animal secretions, drugs or potential toxins develop a significant part of their effect by attaching to this nicotinic ACh receptor.
At muscle-type N M of the receptor in the membrane of muscle fibers of a neuromuscular junction , for example, the blocked tubocurarine preparation curare of vine plants (about Chondodendron tomentosum ) as a competitive antagonist reversible, the addition of ACh to the receptor, the α- bungarotoxin in the saliva of Venomari as Kraits ( e.g. Bungarus multicinctus ) irreversible. In both cases, striated muscles are withdrawn from activation by neuronal impulses. The arrow poison curare was also used for medicinal purposes because of this effect. Contemporary muscle relaxants include cisatracurium , pancuronium and rocuronium as blocking, so-called non-depolarizing muscle relaxants ( benzylisoquinolines or steroid derivatives). Suxamethonium (succinylcholine), on the other hand, is an agonistic substance; it binds to the ACh receptor, activates it and temporarily opens the ion channel by controlling the ligands, as a so-called depolarizing muscle relaxant. This drug also causes muscle paralysis, but via a depolarization block and therefore with preceding muscle twitching. The subsequent desensitization of the receptor cannot be reversed without an interim ligand-free state, i.e. the occupied receptor cannot be promptly reactivated. For the duration of action of succinylcholine, its breakdown by a pseudocholinesterase is primarily decisive (not by acetylcholinesterase, as is the case with ACh).
The neuron type N N of the receptor in the membrane of nerve cells can be specifically blocked by means of hexamethonium or pentamethonium. In the past, these drugs were used as ganglion blockers because, among other things, they interrupt the transmission of excitation from pre- to postganglionic neurons in the ganglia of the peripheral autonomic nervous system; Today, however, such use is obsolete because of the numerous side effects. The alkaloids nicotine (for example in tobacco plants ), coniine (for example in hemlock ) and cytisine (for example in laburnum ) activate this neuronal ACh receptor and are highly toxic (cardiac arrest, respiratory paralysis) because of their strong effect on the autonomic nervous system in the appropriate dose.
Medical importance
In the clinical picture of myasthenia gravis , the body produces autoantibodies against the nicotinergic ACh receptor of the muscular type and therefore leads to muscle weakness. The following is a list of the possible receptor subunits, the position of their gene in the genome and possible clinical pictures if the same is mutated .
| UE | Type | UniProt | Gene locus | OMIM | pathology |
|---|---|---|---|---|---|
| α 1 | muscle | P02708 | 2q24-q32 | 100690 | Myasthenia gravis; Multiple Pterygium Syndrome , Lethal Type; myasthenic syndrome (SCCMS, FCCMS) |
| α 2 | Neuron | Q15822 | 8p21 | 118502 | Nocturnal frontal lobe epilepsy (ENFL4) |
| α 3 | Neuron | P32297 | 15q24 | 118503 | Autoimmune autonomic ganglionopathy; Susceptibility to lung cancer (LNCR2); Susceptibility to Peripheral Arterial Disease (PAOD2) |
| α 4 | Neuron | P43681 | 20th | 118504 | Nocturnal frontal lobe epilepsy (ENFL1) |
| α 5 | Neuron | P30532 | 15q24 | 118505 | Lung Cancer Susceptibility (LNCR2) |
| α 6 | Neuron | Q15825 | 8p22 | 606888 | |
| α 7 | Neuron | P36544 | 15q13.3 | 118511 | |
| β 1 | muscle | P11230 | 17p12-p11 | 100710 | Myasthenic Syndrome (SCCMS, ACHRDCMS) |
| β 2 | Neuron | P17787 | 1q21.3 | 118507 | Nocturnal frontal lobe epilepsy (ENFL3) |
| β 3 | Neuron | Q05901 | 8th | 118508 | |
| β 4 | Neuron | P30926 | 15q24 | 118509 | |
| γ | P07510 | 2q33-34 | 100730 | Escobar Syndrome ; Multiple pterygium syndrome , lethal type | |
| δ | Q07001 | 2q33-qter | 100720 | Multiple Pterygium Syndrome , Lethal Type; myasthenic syndrome (SCCMS, FCCMS) | |
| ε | selected muscle | Q04844 | 17p13.2 | 100725 | Myasthenia gravis; myasthenic syndrome (SCCMS, FCCMS, ACHRDCMS) |
See also
Web links
Individual evidence
- ^ V. Itier, D. Bertrand: Neuronal nicotinic receptors: from protein structure to function . In: FEBS letters . tape 504 , no. 3 , August 31, 2001, p. 118-125 , doi : 10.1016 / s0014-5793 (01) 02702-8 , PMID 11532443 .
- ↑ See Shepard, 2004: The synaptic organization of the brain .
- ↑ Elisabeth P. Golden, Steven Vernino: Autoimmune autonomic neuropathies and ganglionopathies: epidemiology, pathophysiology, and therapeutic advances . In: Clinical Autonomic Research . May 15, 2019, doi : 10.1007 / s10286-019-00611-1 .