Rocuronium
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
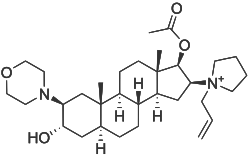
|
||||||||||||||||
| Counterion not shown | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Rocuronium | |||||||||||||||
| other names |
[3-Hydroxy-10,13-dimethyl-2-morpholin-4-yl-16- (1-prop-2-enyl-2,3,4,5-tetrahydropyrrol-1-yl) -2,3,4 , 5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1 H -cyclopenta [a] phenanthren-17-yl] acetate ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 32 H 53 N 2 O 4 + | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 529.77 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rocuronium is a non-depolarizing muscle relaxant that was introduced into clinical anesthesia in Germany in 1995 . It is a further development of the aminosteroid Vecuronium .
properties
Among the non-depolarizing muscle relaxants, rocuronium has the shortest onset of action (attack time) and is therefore an alternative if succinylcholine can not be used due to contraindications during rapid sequence induction . Intubation conditions are good after approx. 60–90 seconds . The clinical duration of action (time until 25% spontaneous recovery of the control twitch strength) with 0.6 mg · kg −1 rocuronium bromide is 30–40 minutes. The total duration of action (time of spontaneous recovery to 90% of the control twitch strength) amounts to 50 minutes. In addition, Rocuronium does not release any histamine in doses of 0.6 to 1.2 mg / kg.
Dismantling
Rocuronium is mainly broken down in the liver and partly excreted via the kidneys . Caution is therefore advised in the event of impaired liver function.
application
Like all muscle relaxants, Rocuronium does not have a sedative effect and may therefore only be used during anesthesia . It relaxes all skeletal muscles, therefore the administration of Rocuronium leads to temporary paralysis of the respiratory muscles and apnea . The application therefore requires the availability of a ventilation option and the presence of a doctor who is experienced in airway management .
Antidote
Sugammadex, an antidote specific to rocuronium, has been available since mid-2008 . With Sugammadex, the muscle-blocking effect of rocuronium can be ended within a few minutes, which makes the use of rocuronium for induction of anesthesia with difficult airway management such. B. the RSI (see above) makes it safer.
Trade names
Esmeron (D, A, CH), as well as generic (D, A)
literature
- JM Hunter: Rocuronium: the newest aminosteroid neuromuscular blocking drug. In: British journal of anesthesia. Volume 76, Number 4, April 1996, pp. 481-483, PMID 8652315 . (Review).
- WC Bowman: Neuromuscular block. In: British journal of pharmacology. Volume 147 Suppl 1, January 2006, pp. S277-S286, doi : 10.1038 / sj.bjp.0706404 . PMID 16402115 . PMC 1760749 (free full text). (Review).
- T. Raghavendra: Neuromuscular blocking drugs: discovery and development. In: Journal of the Royal Society of Medicine. Volume 95, Number 7, July 2002, pp. 363-367, PMID 12091515 . PMC 1279945 (free full text).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , p. 37.
- ↑ M. Naguib: Sugammadex: Another milestone in clinical neuromuscular pharmacology. In: Anesthesia and analgesia. Volume 104, Number 3, March 2007, pp. 575-581, doi : 10.1213 / 01.ane.0000244594.63318.fc , PMID 17312211 .