Cisatracurium
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
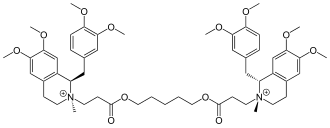
|
||||||||||||||||||||||
| Counterion not shown | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cisatracurium besylate | |||||||||||||||||||||
| other names |
2,2 '- [Pentane-1,5-diylbis (oxycarbonylethylene)] bis - [(1 R , 2 R ) -1- (3,4-dimethoxybenzyl) -6,7-dimethoxy-2-methyl-1, 2,3,4-tetrahydroisoquinolinium] dibenzenesulfonate ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cisatracurium , also Cis-Atracurium , is a drug introduced in Germany in 1996 from the group of non-depolarizing muscle relaxants . It corresponds to the cis - cis - isomer of the racemate atracurium and is contained in this to about 15%. Like other non-depolarizing muscle relaxants, it works by competitive inhibition of acetylcholine at the nicotinic acetylcholine receptor .
Application areas (indications)
Cisatracurium is used to relax the muscles (relax the muscles) for surgery or other medical procedures, such as: B. to achieve mechanical ventilation . It is not suitable for Rapid Sequence Induction because of its average attack time (see below).
Pharmacodynamics
The mean ED95 (i.e. the dose that causes a 95 percent suppression of the response of the adductor pollicis muscle when the ulnar nerve is stimulated ) is 0.05 mg / kg body weight (for comparison: the ED95 of the racemate atracurium is 0, 17 mg / kg under otherwise comparable conditions). With the double to quadruple ED95, i. H. 0.1-0.2 mg / kg body weight, the time to maximum effect ( attack time ) is in the middle range with three to five minutes. The duration of action is then also in the medium range at 30 to 80 minutes.
In contrast to atracurium, it leads to a significantly lower release of histamine. 80–90% of its degradation occurs through Hofmann elimination and only to a small extent through unspecific esterases in the plasma . This means that it can also be used well in patients with liver or kidney disorders, in whom other muscle relaxants can have significantly longer effects due to the reduced excretion.
The levels of the potentially toxic laudanosine achieved are lower than those of atracurium. As a drug Cisatracurium is used primarily as benzenesulfonic acid - salt ( besilate used).
Antagonization
Specific antagonization is not possible. If necessary, cholinesterase inhibitors such as neostigmine can indirectly increase its concentration in the synaptic gap and thus on the motor endplate by inhibiting the breakdown of acetylcholine. This displaces the muscle relaxant again from the receptor, which enables a normal physiological course of muscle excitation.
Chemical properties
The benzyl isoquinoline derivative cisatracurium chemically belongs to the esters , tetrahydropyridine derivatives and phenol ethers . It is a cis - cis - isomer of the racemate atracurium .
Trade name
The trade name Nimbex was designed by Burroughs Wellcome (now GlaxoSmithKline ). It stands for "excellent neuromuscular blockers" (Engl. Ex cellent N € m uscular b loose).
literature
- Liu M, Dilger JP: Site selectivity of competitive antagonists for the mouse adult muscle nicotinic acetylcholine receptor . In: Molecular Pharmacology . 75, No. 1, January 2009, pp. 166-173. PMID 18842832 . PMC 2606922 (free full text).
- Demazumder D, Dilger JP: The kinetics of competitive antagonism of nicotinic acetylcholine receptors at physiological temperature . In: The Journal of Physiology . 586, No. 4, February 2008, pp. 951-963. PMID 18063662 . PMC 2375649 (free full text).
- Dilger JP, Vidal AM, Liu M, et al. : Roles of amino acids and subunits in determining the inhibition of nicotinic acetylcholine receptors by competitive antagonists . In: Anesthesiology . 106, No. 6, June 2007, pp. 1186-1195. PMID 17525594 . PMC 2367005 (free full text).
- Kopman AF, Zank LM, Ng J, Neuman GG: Antagonism of cisatracurium and rocuronium block at a tactile train-of-four count of 2: should quantitative assessment of neuromuscular function be mandatory? . In: Anesthesia and Analgesia . 98, No. 1, January 2004, pp. 102-106, table of contents. PMID 14693596 .
Web links
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of (1R, 1'R, 2R, 2'R) -2,2 '- (3,11-dioxo-4,10-dioxatridecamethylene) to (1,2,3 , 4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 28, 2019.
- ↑ a b Notes on drugs.com
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , p. 37.
- ↑ Fodale V, Santa Maria LB: Laudanosine to atracurium and cisatracurium metabolites . In: Eur J Anaesthesiol . 19, No. 7, July 2002, pp. 466-473. PMID 12113608 .
