Amino alcohols
| Amino alcohols |
|---|
|
|
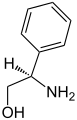
| General structure of the (primary) amino alcohols with the primary amino and hydroxyl groups marked in blue . The radical R represents an aliphatic , cyclic or aromatic radical. |
In organic chemistry, amino alcohols , also alkanolamines , form a group of compounds that contain at least two functional groups , one of which is a hydroxyl group and an amino group . As a rule, one speaks of primary amino alcohols in which the compound has a primary amino group (-NH 2 ).
The term alkanolamines is a technical, trivial term , amino alcohols as the systematic name of the group of substances.
N -alkylated amino alcohols
Amino alcohols whose compounds have a secondary (-NHR) or tertiary (-NR 2 ) amino function are referred to as alkylalkanolamines or alkylamino alcohols . Alkylalkanolamines are produced by alkylating the corresponding epoxides . Primary and secondary amines are used as alkylating agents and the corresponding N -alkylated derivatives of the primary amino alcohols are formed.
Classification
Depending on the relative position (“distance”) of the hydroxy and amino groups to one another, a distinction is made between
- α-amino alcohols [1,1-amino alcohols, usually called hemiaminals (synonym: hemiaminoacetal)],
- β-amino alcohols (1,2-amino alcohols),
- γ-amino alcohols (1,3-amino alcohols) and
- δ-amino alcohols (1,4-amino alcohols) etc.
Relative stability
- α-Amino alcohols: hemiaminals can be stable if no hydrogen atom is bonded to the nitrogen atom. They often cannot be observed directly. This usually requires special conditions. If a hydrogen atom is bonded to the nitrogen atom of a hemiaminal, water is usually split off with the formation of an imine .
- β-amino alcohols, γ-amino alcohols and δ-amino alcohols, etc. are generally stable.
Manufacturing
1,1-amino alcohols
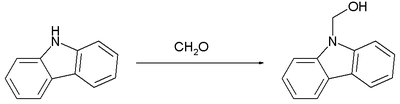
The following reaction shows an example of the formation of a hemiaminal from the secondary amine carbazole and formaldehyde .
The formation of hemiaminales is a key reaction in the asymmetric synthesis of saxitoxin .
1,2-amino alcohols
The simplest amino alcohol, 2-aminoethanol (colamine), can be represented by the reaction of oxirane with ammonia or by the reaction of aziridine with water. Ring opening of an oxirane with an azide leads to an azido-substituted alcohol , the subsequent reduction of which leads to the corresponding 1,2-amino alcohol.
By reduction of enantiomerically pure Aminosäureestern with lithium aluminum hydride can be prepared in high yield optically active amino alcohols synthesize. The reduction of amino acids with borane (BH 3 ) also produces amino alcohols. The chiral 1,2-amino alcohols - derived from α-amino acids by reduction - are often referred to by trivial names resulting from the amino acid nomenclature, e.g. B .:
- ( S ) - proline gives ( S ) - prolinol
- ( S ) - alanine gives ( S ) - alaninol
- ( S ) - valine gives ( S ) - valinol
- ( S ) - Leucine gives ( S ) - Leucinol
- ( S ) - Phenylalanine gives ( S ) - Phenylalaninol
- ( R ) - phenylglycine gives ( R ) - phenylglycinol
The reaction of enantiomerically pure amino acid esters with Grignard compounds yields amino alcohols that are also tertiary alcohols with retention of configuration .
1,3-amino alcohols
When ring opening of an oxetane with an amine, a 1,3-amino alcohol can be formed, but the reaction is not as smooth as the analogous ring opening of an oxirane with an amine.

use
Technically important amino alcohols are mono-, di- and triethanolamine, dimethylaminoethanol , diethylaminoethanol, N -methyldiethanolamine and mono-, di- and triisopropanolamine. Alkanolamines can be used in the gas cleaning to hydrogen sulfide - and carbon dioxide - absorption can be used. Here, the acid gases mentioned first dissolve in an aqueous alkanolamine solution. Then they react with the amino group of the alkanolamine.
Medicinal substances
Numerous drugs belong to the 2-amino alcohols. One example is the muscle relaxant phenyramidol. Most β-blockers belong to the amino alcohols, such as. B. Propanolol , Acebutolol , Atenolol , Betatoxol, Bisoprolol, Carteolol , Nebivolol , Labetalol , Metoprolol , Pindolol , Timolol and Penbutolol.
Catalysts in Organic Synthesis
In the catalytic enantioselective addition of organozinc to aldehydes , chiral amino alcohols are used as catalysts . Chiral 1,2-amino alcohols are used to produce oxazaborolidines ( CBS catalysts ), which are used as catalysts in the enantioselective reduction ( CBS reduction ) of asymmetrical ketones to secondary alcohols. The names of these amino alcohols are derived from the trivial names of the amino acids from which they were synthesized:
Individual evidence
- ↑ Entry on alkanolamines. In: Römpp Online . Georg Thieme Verlag, accessed on October 6, 2019.
- ↑ Stabilization of Labile Carbonyl Addition Intermediates by a Synthetic Receptor Tetsuo Iwasawa, Richard J. Hooley, Julius Rebek Jr. Science 317, 493 ( 2007 ) doi : 10.1126 / science.1143272 .
- ↑ Milata Viktor, Kada Rudolf, Lokaj Jn: Carbazol -9-yl-methanol. In: Molbank. 2004, 2004, p. M354, doi : 10.3390 / M354 .
- ↑ Reaction in refluxing methanol with sodium carbonate . Acid catalysis reacts the amino acetal to the imine N, N´-biscarbazol-9-yl-methane.
- ↑ (+) - Saxitoxin: A First and Second Generation Stereoselective Synthesis James J. Fleming, Matthew D. McReynolds, and J. Du Bois J. Am. Chem. Soc. , 129 (32), 9964-9975, 2007 . doi : 10.1021 / ja071501o .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 502, ISBN 3-342-00280-8 .
- ↑ Michael B. Smith: March's advanced organic chemistry , John Wiley & Sons, 7th edition, 2013, p. 489, ISBN 978-0-470-46259-1 .
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, p. 85, 1982, ISBN 3-527-25892-2 .
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organische Chemie , Springer Spectrum, 2013, 2nd edition, p. 1212. ISBN 978-3-642-34715-3 .
- ↑ Michael B. Smith: March's advanced organic chemistry , John Wiley & Sons, 7th edition, 2013, p. 490, ISBN 978-0-470-46259-1 .
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , p. 118.
- ↑ Kohl, AL, Nielsen, RB: Gas Purification , Gulf Publ. Co., Houston, TX, 5th ed., 1997.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organische Chemie , Springer Spectrum, 2013, 2nd edition, pp. 772–773. ISBN 978-3-642-34715-3 .
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; Pp. 7321-7322, ISBN 978-0-911910-00-1 .
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th edition, de Gruyter Verlag, Berlin, 2011, pp. 351–352, ISBN 978-3-11-024894-4 .
- ↑ Thomas Mehler, Jürgen Martens : New Thioether Derivatives as Catalysts for the Enantioselective Addition of Diethylzinc to Benzaldehyde , In: Tetrahedron: Asymmetry 1994, 5 , 207-210, doi : 10.1016 / S0957-4166 (00) 86174-1 .
- ↑ Sabine Wallbaum, Jürgen Martens: Catalytic Enantioselective Addition of Diethylzinc to Aldehydes: Application of a new Bicyclic Catalyst, Tetrahedron: Asymmetry 1993, 4 , 637-640, doi : 10.1016 / S0957-4166 (00) 80167-6 .
- ↑ Viola Peper, Jürgen Martens: New β-Amino Alcohols as Chiral Ligands for the Catalytic Enantioselective Reduction of Prochiral Ketones and the Nucleophilic Addition of Diethylzinc to Benzaldehyde , Chemical Reports 1996, 129 , 691–695, doi : 10.1002 / cber.19961290616 .
- ↑ Sabine Wallbaum and Jürgen Martens: Asymmetric Syntheses with Chiral Oxazaborolidines , Tetrahedron: Asymmetry 3 (1992) pp. 1475-1504, DOI: 10.1016 / S0957-4166 (00) 86044-9 .