Oxetane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Oxetane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 6 O | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 58.08 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.89 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−97 ° C |
||||||||||||||||||
| boiling point |
48 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
soluble in water (681 g l −1 at 25 ° C) |
||||||||||||||||||
| Refractive index |
1.3961 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−110.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Oxetane is an organic compound . Oxetane is the simplest representative of the heterocyclic four-rings with an oxygen atom in the ring and is therefore one of the cyclic ethers .
Oxetane is a reactive, colorless, highly volatile liquid.
synthesis
By reacting 3-chloropropyl acetate with potassium hydroxide at approx. 140 - 150 ° C.
However, the yield is only a little over 40% because a number of by-products are formed.
Another possibility is to cyclize 3-chloropropan-1-ol with a strong base.
The yield is even lower than in the first reaction.
Another possibility is to produce oxetanes using a Paternò-Büchi reaction .
Reactivity
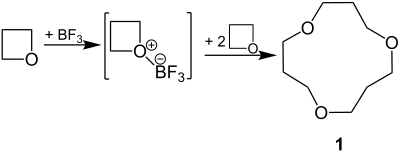
Lewis acids such as boron trifluoride (BF 3 ) can be added to a non-bonding electron pair on the O atom of the oxetane. Cyclooligomerization then takes place in dichloromethane as solvent. The main product is cyclotrimer 1 :
Linear polyethers are formed under different reaction conditions, especially in the presence of water.
See also
Individual evidence
- ↑ Oxetane data sheet (PDF) from Merck , accessed on January 18, 2011.
- ↑ a b c d e f g h i Entry on 1,3-epoxypropane in the GESTIS substance database of the IFA , accessed on December 11, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-408.
- ↑ Entry on 1,2-epoxypropane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ↑ a b C. R. Noller: Trimethylene Oxide In: Organic Syntheses . 29, 1949, pp. 92-93, doi : 10.15227 / orgsyn.029.0092 ; Coll. Vol. 3, 1955, p. 835 ( PDF ).
- ↑ a b Theophil Eicher , Siegfried Hauptmann, Andreas Memory: The Chemistry of Heterocycles, Wiley-VCH, 2012, ISBN 978-3-527-32747-8 , pp 45-48.