Aziridine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aziridine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 5 N | ||||||||||||||||||
| Brief description |
colorless liquid with an ammonia-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 43.07 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.83 g cm −3 |
||||||||||||||||||
| Melting point |
−71 ° C |
||||||||||||||||||
| boiling point |
approx. 55 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
miscible with water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aziridine or azacyclopropane is a chemical compound from the group of heterocyclic secondary amines . The name ethyleneimine, which is also used, suggests that there is actually no imine group (H 3 CHC = NH) in the molecule.
properties
Physical Properties
It is a colorless, water-soluble, easily mobile, volatile liquid with an ammonia-like odor.
Chemical properties
Aziridine is highly flammable and the vapors can form explosive mixtures with air. Small amounts of acids, acid-releasing compounds and even the normal carbon dioxide content of the air can lead to a highly exothermic, possibly explosive polymerisation , even in the case of stabilized products . The heat of decomposition determined by DSC is −87 kJ mol −1 or −2020 kJ kg −1 .
Health hazards
Aziridine is acutely toxic when inhaled, swallowed and in contact with skin. The vapors have a strong irritant effect on the mucous membranes, stimulate the central nervous system and damage the kidneys. Acute symptoms are reddening, blistering and necrosis of the skin and mucous membranes, corneal opacity, bronchopneumonia, pulmonary edema and shortness of breath. Long-term carcinogenic effects are assumed. Allergies to aziridines are increasingly being noted (type 1 and type 3 allergies: asthma , allergic rhinitis , allergic contact dermatitis and urticaria ). Residual monomers in the formulation of polyfunctional aziridines and also PFAs themselves can cause allergies .
Extraction and presentation
The first production succeeded in 1899 Willy Marckwald by treating β-haloamines with caustic soda .
Monoethanolamine process
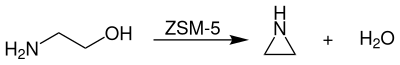
Aziridine is industrially produced by dehydration of monoethanolamine at temperatures of 350-450 ° C and reduced pressure of modified ZSM-5 zeolite catalysts prepared.
The process is usually carried out in the gas phase in a flow tube. Efforts have already been made since the 1970s to develop a suitable catalyst for this process so that it takes place economically and with high yield . Finally, in 1990, the Nippon Shokubai company commissioned the first plant for the industrial production of aziridine using the monoethanolamine process. The prerequisite for this was the successful development of a catalyst system with which a selectivity of 90% with conversions of 40–80% could be achieved. The product mixture then has to be separated by multi-stage distillation ; unreacted monoethanolamine is returned to the reactor.
Wenker trial
Another method is the Wenker synthesis , in which monoethanolamine is first esterified with sulfuric acid and then treated with sodium hydroxide solution:
However, the atom economy of this method is poor, which is why the Wenker synthesis is a purely laboratory process for the production of small amounts of aziridine.
Derivatives and Uses
Aziridine can be polymerized to give polyethyleneimine , but the direct polymerization results in a highly branched polymer. Linear polyethyleneimine can be produced via 2-alkyl-substituted 2- oxazolines . Polyethyleneimine is used as a reagent for transfection , as a precipitation reagent or (if necessary after reaction with epichlorohydrin ) in paper production as a wet strength and retention agent.
Aziridine derivatives such as mitomycin C , triaziquon and thiotepa have been used as alkylating cytostatics for the treatment of cancer diseases such as breast cancer , bladder cancer and ovarian cancer for about 50 years . However, the importance of these active ingredients has decreased significantly as they have significant side effects.
Derivatives such as polyfunctional aziridines (PFA) can be used as crosslinkers for water-soluble resins.
Web links
Individual evidence
- ↑ a b c d e f g h i j Entry on ethyleneimine in the GESTIS substance database of the IFA , accessed on December 15, 2019(JavaScript required) .
- ↑ Entry on aziridines in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 151-56-4 or aziridine ), accessed on November 2, 2015.
- ↑ Grewer, T .; Klais, O .: Exothermic decomposition - investigations of the characteristic material properties , VDI-Verlag, series "Humanisierung des Arbeitsleben", Volume 84, Düsseldorf 1988, ISBN 3-18-400855-X , p. 9.
- ↑ a b Public Health Service, Baden-Württemberg ( Memento from February 15, 2015 in the Internet Archive ).
- ↑ a b Ulrich Steuerle, Robert Feuerhake: Aziridines. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., December 15, 2006, doi : 10.1002 / 14356007.a03_239.pub2 .
- ↑ Blandine Brissault, Antoine Kichler, Christine Guis, Christian Leborgne, Olivier Danos, Hervé Cheradame: Synthesis of Linear Polyethyleneimine Derivatives for DNA Transfection . In: Bioconjugate Chemistry . tape 14 , no. 3 , 2003, p. 581-587 , doi : 10.1021 / bc0200529 .
- ↑ Use of polyfunctional aziridines .