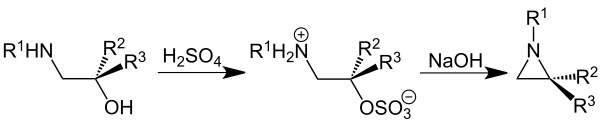
Wenker synthesis
In the Wenker synthesis , a beta-amino alcohol is converted into an aziridine by reaction with sulfuric acid and then with sodium hydroxide solution :
The synthesis takes place in two steps. In the first step, the β-amino alcohol is reacted with sulfuric acid at a high temperature (250 ° C). A sulfuric acid ester is formed as an intermediate. The aziridine is then obtained by using a base (sodium hydroxide solution) with internal molecular ring closure.
In a modified variant at a lower temperature (140 to 180 ° C) a better yield is obtained.
Web links
- Wenker synthesis in the portal for organic chemistry
Individual evidence
- ^ Henry Wenker: The Preparation of Ethylene Imine from Monoethanolamine . In: Journal of the American Chemical Society . 57, No. 1, 1935, pp. 2328-2328. doi : 10.1021 / ja01314a504 .
- ↑ Philip A. Leighton, William A. Perkins, and Melvin L. Renquist: A Modification of Wenker's Method of Preparing Ethyleneimine J. Am. Chem. Soc. ; 69 (6). 1947, pp. 1540-1540, ( doi : 10.1021 / ja01198a512 ).