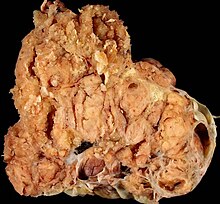
Ovarian cancer
| Classification according to ICD-10 | |
|---|---|
| C56 | Malignant neoplasm of the ovary |
| ICD-10 online (WHO version 2019) | |
The ovarian cancer or ovarian cancer is a malignant disease of the ovaries . In the western world, after endometrial and cervical cancer, it is the third most common genital cancer in women and has a worse prognosis than that.
Epidemiology
The mean age of onset in Germany is 69 years, although much younger women can also get the disease, often in connection with genetic predisposition . Women in Germany have a lifetime risk of 1.5% of developing ovarian cancer. The incidence of ovarian cancer has decreased significantly in the last 20 years, whereas the mortality rates are at a roughly constant level. From 2005 to 2009, the age- and population-corrected incidence rates across Germany fell from 13.5 to 11.5 new cases per 100,000 female residents per year. The estimated incidence rates for 2012 suggest 7,200 new cases, which corresponds to an age- and population-adjusted incidence rate of 11.0 new cases per 100,000 female residents.
causes
So far, numerous mutations, such as the duplication and loss of chromosome segments in the cells involved, which are distributed across the entire genome , have been identified as triggers for this type of tumor . The disease occurs in families. The two genes BRCA1 and BRCA2 play a role (as in breast cancer ). Childless and late births have a 2.5 times higher risk of developing it than the normal population. Hormonal contraceptives , frequent pregnancies and long breastfeeding , on the other hand, are protective factors as they "immobilize" the ovaries.
Symptoms
The symptoms are often non-specific, such as B. gastrointestinal complaints, reduced performance or bleeding disorders, so that the tumors are often only recognized at an advanced stage. Genital bleeding is reported in 25% of cases.
early detection
There are no effective methods for early detection. There is no evidence for the benefit of annual screening using transvaginal ultrasound and CA-125 determination, even in high-risk situations .
According to an online survey in 2018 on behalf of the MDS, the ultrasound of the ovaries for early cancer detection is the most common individual health service (IGeL) in women and the second most common IGeL overall. The MDS IGeL Monitor rates this examination as “negative”, as several studies have shown that as many women die of ovarian cancer with an ultrasound examination as without an examination. On the other hand, women are often unnecessarily alarmed by false alarms and even healthy ovaries are removed.
Diagnosis
The transvaginal ultrasound, in which an enlarged ovary can be detected, and the determination of CA-125 and CA 19-9 are pioneering in ovarian cancer . Afterwards, a computed tomography or an MRI can be helpful (also for surgical planning).
pathology
Ovarian carcinomas start from the epithelial tissue of the ovaries:
- Serous papillary cystadenocarcinoma (40% of ovarian malignancies)
- endometroid carcinoma (20%)
- Mucinous cystadenocarcinoma (10%)
- Clear cell carcinoma (5%)
- Solid ovarian cancer (rare)
There are also benign tumors of the epithelium, which are not carcinomas, but can be precursors to malignant neoplasms. The likelihood of malignant degeneration varies greatly from person to person.
- Serous cystadenoma (30%)
- Mucinous cystadenoma (15%)
- Endometrioid tumors (5%)
- Brenner tumor (2%)
- Adenomatoid tumor (very rare)
- Cystadenofibroma
The list only contains those tumors that histologically originate from the epithelium. However, benign and malignant neoplasms of other origins can also be found in the ovaries. These include tumors of the sexually differentiated and undifferentiated gonadal mesenchyme as well as germ cell tumors ( teratoma and gonadoblastoma ).
Mucinous ovarian cancer
Mucinous ovarian cancer (MOC) is much rarer than serous. It differs significantly from other ovarian cancers in many ways. Despite its size, it is diagnosed in stage FIGO I in 80% of cases and therefore has a much better prognosis than serous ovarian carcinoma. The gene expression profile also differs significantly. In particular, galectin-4 (LGALS4) is strongly and specifically expressed in mucinous ovarian carcinomas. Expression is lower in benign mucinous cysts and borderline tumors . This supports the tumor progression model of the development of benign mucinous cysts via atypical proliferation to invasive carcinoma. LGALS4 should be useful as an early marker for mucinous ovarian cancer. Common tumor markers are CEA or CA19-9, while in serous ovarian cancer CA125 predominates. The average age of the patients is also significantly lower. A SEER analysis (Surveillance, Epidemiology, and End Results) found an age of less than 44 years in 26% of mucinous ovarian carcinomas. The mean age was 53 years for mucinous and 61 years for serous ovarian cancer. Another difference is sensitivity to platinum-based chemotherapy. While over 70% of serous ovarian carcinomas respond to this chemotherapy, it is only 20% to 60% of mucinous ovarian carcinomas. The development of mucinous ovarian cancer shows parallels to the development of colorectal cancer. There is a KRAS mutation in 40% to 75% . Also HER2 - amplification or TP53 mutation come early before the development of mucinous carcinomas.
The ovary can also be a metastatic target for other tumors. 10% of all ovarian tumors are metastatic tumors. The most common tumors of the digestive tract (30% to 70%) such as the Krukenberg tumor (metastasis of a gastric cancer ), tumors of the breast (10% to 30%) and the uterus (also 10% to 30%). The tumor marker CA-125 belongs to v. a. to serous, CA 19-9 to mucinous ovarian cancer. Another relevant tumor marker is CA 72-4 .
Stages
The stages of the FIGO classification in ovarian cancer practically correspond to the 'T' value of the TNM classification .
Stages according to TNM classification and FIGO (Fédération Internationale de Gynécologie et d'Obstétrique):
| TNM | FIGO | criteria |
|---|---|---|
| T1 | I. | Tumor limited to ovaries |
| 1a | IA |
|
| 1b | IB |
|
| 1c | IC |
|
| T2 | II | Tumor affects one or both ovaries and spreads to the pelvis |
| 2a | IIA | Spread to and / or implants on the uterus and / or fallopian tubes |
| 2 B | IIB | Spread to other pelvic tissues |
| 2c | IIC |
|
| T3 | III |
|
| 3a | IIIA | microscopic peritoneal metastases beyond the pelvis |
| 3b | IIIB |
|
| 3c | IIIC |
|
| Nx | No statement can be made on regional lymph node metastases. | |
| N0 | No metastases in the regional lymph nodes. | |
| N1 | Metastases in the regional lymph nodes. | |
| M0 | No distant metastases detectable. | |
| M1 | IV | The tumor has formed distant metastases (except for peritoneal metastases). |
According to new genetic studies, ovarian cancer in its advanced stage can be divided into four different subtypes due to the different pattern of gene activities of tumor cells. A “cancer genome atlas” now available for this type of tumor is intended to enable therapies tailored to the respective subtype in the future.
therapy
Treatment usually consists of a combination of surgery and adjuvant chemotherapy adapted to the stage of the disease . Antibody therapy has also been available for patients in the advanced stages of the disease since December 2011.
surgery
The operation serves on the one hand to secure the diagnosis and to determine the exact stage . The entire abdomen is systematically examined for cancer using a median laparatomy . In addition, tissue samples are taken for histological assessment. On the other hand, the operation aims to remove all visible cancerous ulcers as completely as possible. The radicality of the tumor reduction (as the only influenceable prognostic factor) significantly determines the healing prospects. To ensure that as few cancer cells as possible remain in the body, the standard operation includes the removal of the ovaries and fallopian tubes , the uterus , the large network and the lymph nodes . Depending on the stage of the disease, it may be necessary to remove other parts of the organ, such as parts of the intestine. A fertility-preserving operation is only possible in a confirmed early stage (FIGO stage IA) . The uterus and the unaffected ovary are preserved if the child wishes to have children. To treat invisible peritoneal metastases during surgery, there is the option of hyperthermic intraperitoneal chemoperfusion (HIPEC). A warmed solution containing a chemotherapeutic agent (for example cisplatin or mitomycin C ) is distributed in the abdomen over about an hour. As a result, the drug also reaches metastases with poor blood circulation, and the warming can increase its effectiveness.
chemotherapy
Postoperatively , chemotherapy containing platinum is performed as standard . In the early stage of FIGO IA, grade 1, this can be omitted. Up to the FIGO IIA stage z. B. treated with carboplatin . In the advanced stage, a taxane , e.g. B. Paclitaxel , combined. In April 2019, the CHMP of the European Medicines Agency (EMA) issued a positive approval recommendation for the PARP inhibitor olaparib (trade name: Lynparza , manufacturer: AstraZeneca ) for 1st-line therapy . As a rule, a corresponding approval is then obtained from the European Commission.
Antibody therapy
In addition to chemotherapy, the monoclonal antibody bevacizumab has been approved for the treatment of patients with advanced ovarian cancer (FIGO stage IIIB-IV) since December 2011 . The angiogenesis inhibitor binds to tumor growth factors and thus prevents the formation of new blood vessels that are responsible for supplying the tumor with oxygen and nutrients. The mean progression-free survival was prolonged to 14.1 months compared to 10.3 months in the control group.
Relapse therapy
In the event of a recurrence of ovarian cancer, surgical treatment can be given if the primary operation was complete, the general condition is good and there is less than 500 ml of ascites (positive AGO score ). Platinum- sensitive tumors ( relapse after more than 6 months) can then be combined with cisplatin in combination with pegylated liposomal doxorubicin , paclitaxel and gemcitabine . As an alternative, doxorubicin with trabectedin is available. In the case of early relapses under 6 months (platinum resistance ), "maintaining quality of life should be in the foreground compared to other therapeutic goals ". Combination therapy offers no advantage here. Monotherapy with pegylated liposomal doxorubicin, topotecan, gemcitabine or paclitaxel is recommended.
In relapsed platinum-sensitive high grade serous ovarian cancer, therapy with olaparib can increase progression-free survival (PFS). The prerequisite for therapy is a. evidence of a BRCA mutation in the tumor or in the germline, which applies to about 20% of patients. In March 2017, the US Food and Drug Administration ( FDA) approved another PARP inhibitor for the treatment of ovarian cancer and related malignancies, niraparib (trade name: Zejula , manufacturer: Tesaro ). The European Commission (EC) approved Zejula (niraparib) in the European Union EU in November 2017 . Zejula is the first oral, once-daily PARP inhibitor approved in Europe that does not require testing for BRCA mutation status or other biomarker testing .
forecast
Decisive for the prognosis are the tumor stage at the time the diagnosis was made, the histological findings and, above all, the size of the residual tumor after the operation.
The prognosis of ovarian cancer is very inconsistent and fluctuates depending on the tumor stage at the time of diagnosis, but also depending on the histological findings and the amount of tumor residue left behind during the first operation. The 5-year survival rate (averaged over all patients) is 30–40%. The main reason for this is the mostly late diagnosis and the high risk of recurrence.
| stage | 5 year survival rate |
| FIGO I | 80% |
| FIGO II | 60% |
| FIGO III | 23% |
| FIGO IV | 14% |
literature
- S3 guideline : Diagnostics, therapy and aftercare of malignant ovarian tumors Version 2.1 - November 2017, AWMF register number: 032 / 035OL, full text (PDF) as of 9/2017, valid until 11/2020
- Alexander Burges, Barbara Schmalfeldt: Ovarian Carcinoma: Diagnosis and Therapy . In: Dtsch Arztebl Int . No. 108 (38) , 2011, pp. 635-641 ( review ).
- Cancer in Germany 2013/2014. 11th edition. Robert Koch Institute and the Society of Epidemiological Cancer Registers in Germany V., Berlin 2017, ISBN 978-3-89606-214-7 ;
- Marius Wunderle: Familial breast and ovarian cancer. W. Zuckschwerdt Verlag, Munich 2019, ISBN 978-3-86371-244-0 (Series: Specialist Consultation Hours)
Web links
- www.destatis.de : Federal Statistical Office, official cause of death statistics
- krebsdaten.de : Information on ovarian cancer from the Center for Cancer Registry Data at the Robert Koch Institute
Individual evidence
- ↑ a b D. Bell et al .: Integrated genomic analyzes of ovarian carcinoma. In: Nature. June 30, 2011, No. 474, pp. 609-615, doi: 10.1038 / nature10166
- ↑ IGeL-Monitor, ultrasound of the ovaries for early cancer detection , accessed on October 19, 2018.
- ^ A b Philippe Morice, Sebastien Gouy, Alexandra Leary: Mucinous Ovarian Carcinoma . In: New England Journal of Medicine . tape 380 , no. 13 , March 28, 2019, ISSN 0028-4793 , p. 1256-1266 , doi : 10.1056 / NEJMra1813254 .
- ↑ Lauren C Peres, Kara L Cushing-Haugen, Martin Köbel, Holly R Harris, Andrew Berchuck: Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage . In: JNCI: Journal of the National Cancer Institute . tape 111 , no. 1 , January 1, 2019, ISSN 0027-8874 , p. 60-68 , doi : 10.1093 / jnci / djy071 , PMID 29718305 , PMC 6335112 (free full text) - ( oup.com [accessed April 7, 2019]).
- ^ VA Heinzelmann-Schwarz, M. Gardiner-Garden, SM Henshall, JP Scurry, RA Scolyer: A distinct molecular profile associated with mucinous epithelial ovarian cancer . In: British Journal of Cancer . tape 94 , no. 6 , March 27, 2006, ISSN 0007-0920 , p. 904-913 , doi : 10.1038 / sj.bjc.6603003 , PMID 16508639 , PMC 2361366 (free full text).
- ^ J Alexandre, I Ray-Coquard, F Selle, A Floquet, P Cottu: Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience . In: Annals of Oncology: Official Journal of the European Society for Medical Oncology . tape 21 , no. December 12 , 2010, ISSN 1569-8041 , p. 2377-2381 , doi : 10.1093 / annonc / mdq257 , PMID 20494964 .
- ↑ Kuang-Leei Chang, Ming-Yung Lee, Wan-Ru Chao, Chih-Ping Han: The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma . In: Human Genomics . tape 10 , December 28, 2016, ISSN 1473-9542 , doi : 10.1186 / s40246-016-0096-9 , PMID 28031051 , PMC 5192568 (free full text).
- ↑ LH Sobin, MK Gospodarowicz, Ch. Wittekind: UICC: TNM classification of malignant tumors. 7th edition. Wiley-Blackwell, Oxford 2009, ISBN 978-1-4443-3241-4
- ↑ Ch. Wittekind, H.-J. Meyer: TNM classification of malignant tumors. 7th edition. Wiley-VCH, Weinheim 2010, ISBN 978-3-527-32759-1 .
- ↑ a b Technical information Avastin (PDF; 265 kB), as of 02/2012.
- ↑ a b c guideline for malignant ovarian tumors; Diagnostics, therapy and follow-up care ( memento of the original dated September 23, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF) Ovar Commission of the Working Group for Gynecology Oncology (AGO) e. V.
- ↑ A. Burges, B. Schmalfeldt: Ovarian carcinoma - diagnosis and therapy. In: Dtsch Arztebl Int. 2011; 108 (38), pp. 635-641. doi: 10.3238 / arztebl.2011.0635
- ↑ HIPEC | Frankfurt Hoechst Clinic. Retrieved October 27, 2019 .
- ↑ Lynparza receives positive EU CHMP opinion for 1st-line maintenance treatment of BRCA-mutated advanced ovarian cancer , PM AstraZeneca on April 29, 2019, accessed on May 2, 2019
- ^ RA Burger et al .: Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer . In: N Engl J Med. 2011; 365, pp. 2473-2483; PMID 22204724 . doi : 10.1056 / NEJMoa1104390
- ↑ Niraparib (ZEJULA) , PM FDA of March 27, 2017, accessed November 27, 2017
- ↑ TESARO ANNOUNCES US FDA APPROVAL OF ZEJULA ™ (NIRAPARIB) FOR WOMEN WITH RECURRENT OVARIAN CANCER PM TESARO dated March 27, 2017, accessed on September 19, 2017.
- ↑ TESARO Announces European Commission Approval of ZEJULA® for Women With Recurrent Ovarian Cancer , PM TESARO from November 20, 2017, accessed on November 27, 2017