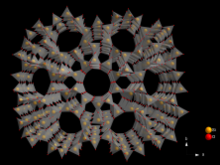
Zeolites (group of substances)
Zeolites are crystalline aluminosilicates that occur naturally in numerous modifications , but can also be produced synthetically . More than 150 different types of zeolite have been synthesized, 60 naturally occurring zeolites are known. The natural zeolites are mineralogically summarized under the term zeolite group .
Chemical composition
The composition of the zeolite group is:
M n + x / n [(AlO 2 ) - x (SiO 2 ) y ] . z H 2 O
- The factor n is the charge of the cation M and is usually 1 or 2.
- M is typically a cation of an alkali or alkaline earth metal . These cations are required to balance the electrical charge of the negatively charged aluminum tetrahedron and are not built into the main lattice of the crystal, but rather stay in the lattice cavities - and are therefore easily movable within the lattice and can also be exchanged afterwards.
- The factor z indicates how many water molecules have been absorbed by the crystal. Zeolites can absorb water and other low-molecular substances and release them again when heated without their crystal structure being destroyed in the process.
- The molar ratio of SiO 2 to AlO 2 or y / x in the empirical formula is called the module . According to the Löwenstein rule, it cannot be less than 1.
Examples of synthetic zeolites:
| Zeolite | Composition of the unit cell |
|---|---|
| Zeolite A | Na 12 [(AlO 2 ) 12 (SiO 2 ) 12 ] · 27H 2 O |
| Zeolite X | Na 86 [(AlO 2 ) 86 (SiO 2 ) 106 ] • 264 H 2 O |
| Zeolite Y | Na 56 [(AlO 2 ) 56 (SiO 2 ) 136 ] • 250 H 2 O |
| Zeolite L | K 9 [(AlO 2 ) 9 (SiO 2 ) 27 ] • 22 H 2 O |
| Mordenite | Na 8.7 [(AlO 2 ) 8.7 (SiO 2 ) 39.3 ] • 24H 2 O |
| ZSM 5 | Na 0.3 H 3.8 [(AlO 2 ) 4.1 (SiO 2 ) 91.9 ] |
| ZSM 11 | Na 0.1 H 1.7 [(AlO 2 ) 1.8 (SiO 2 ) 94.2 ] |
structure
Zeolites consist of a microporous framework structure of AlO 4 - - and SiO 4 - tetrahedra . The aluminum and silicon atoms are connected to one another by oxygen atoms . Depending on the type of structure, this results in a structure of uniform pores and / or channels in which substances can be adsorbed . In nature, water is usually adsorbed there, which can be removed from the pores by heating without changing the zeolite structure. Zeolites can thus be used as sieves, as it were, since only molecules adsorb in the pores which have a smaller kinetic diameter than the pore openings of the zeolite structure. Zeolites therefore also fall into the group of molecular sieves .
Zeolites have a regular arrangement of cavities and channels. Depending on the pore size, one speaks of micro or mesopores . Such materials have an extraordinarily large inner surface , sometimes well over 1,000 square meters per gram. This makes them suitable for a wide range of technical applications, for example as catalysts for numerous chemical industrial processes, as materials for separating chemical substances or as water softeners in detergents.
Zeolites have an anionic framework charge due to trivalent aluminum atoms, to which two divalent oxygen particles can be formally assigned . Therefore, aluminum-containing zeolites contain cations on the inner and outer surfaces . In water-containing zeolite, these cations are often in dissolved form in the channel systems of the zeolites, so they are relatively easily accessible and thus exchangeable. Common cations are Na + , K + , Ca 2+ and Mg 2+ .
Synthetic zeolites are made from strongly alkaline, aqueous solutions of silicon and aluminum compounds. The reactive starting materials used are, for example, sodium waterglass , silica gel or silica as the silicon source and aluminum hydroxide or other aluminum salts as the aluminum source . Which zeolite is formed from the reaction mixture depends on various factors such as the composition of the reaction mixture, the stirring speed and the crystallization temperature. Template effects of organic cations also play an important role in the question of which zeolites actually arise .
Manufacture and modification
For the synthesis of zeolites, alkaline solutions of reactive silicon and aluminum compounds are used , whereby the formation of a reactive gel is a prerequisite. The amorphous reaction mixtures are converted into the crystalline products between 60 and 200 ° C. At temperatures above 100 ° C, work must be carried out under increased pressure. Certain zeolites such as ZSM 5 and ZSM 11 can be used in the presence of organic cations, e.g. B. tetrapropylammonium (C 3 H 7 ) 4 N + , can be produced.
Zeolites can be modified by exchanging the ions or chemical treatment. In the case of zeolites used catalytically , the aim of this modification is , on the one hand, to increase the catalytic effect and, on the other hand, to increase the thermal or chemical resistance. The most common modification is the introduction of metal particles in order to obtain bifunctional, catalytically highly active centers.
Another important treatment is the addition of acids to zeolites . Acid-stable zeolites can be acid-treated directly. This leads to the formation of acidic centers. In order to also generate these in acid-sensitive zeolites, they are often deammonized: First, an alkali cation is exchanged for an ammonium ion , which is then decomposed when heated to around 500 ° C. At the same time, the sample is dealuminated, which stabilizes the structure.
If you replace some silicon atoms with zinc or cobalt atoms and some oxygen bridges with imidazolate bridges, then Zeolitic Imidazolate Frameworks (ZIFs) are created. Some of these ZIFs can selectively store large amounts of carbon dioxide (1 l ZIF-69 stores 82.6 l carbon dioxide at 0 ° C and 1 bar), which is important for carbon dioxide sequestration .
Etymology and history
The name is derived from the ancient Greek ζέω zéō for "boiling" and the ancient Greek λίθος lithos for "stone", meaning "boiling stone". It refers to the lively effervescence (boiling) of the mineral when heated, as bound water is released. The term "zeolite" was coined in 1756 by the Swedish mineralogist Baron Axel Fredrick von Cronstedt .
Zeolites have been used industrially since the mid-1950s, initially as adsorbents and ion exchangers. In 1959, Union Carbide first used Y zeolites as a catalyst component .
At the beginning of the 1970s, zeolites were developed with properties that were completely unknown until then. In 1972, employees of a laboratory at Mobil Oil (formerly Socony = Standard Oil Company of New York) - today part of ExxonMobil - succeeded in creating the basis for a whole series of new zeolites known as pentasils . The most important representative of the Pentasile is the "ZSM-5" ( ZSM stands for zeolithe socony mobil ).
In the 1980s, the chemical company Henkel first used zeolites as a phosphate substitute for washing (see also zeolite A ). It was possible to reduce water eutrophication , but it soon became apparent that zeolites lead to damage in sewage water purification. Therefore one tries now to replace these more with silicates .
use
Zeolites have a wide range of possible uses, including as ion exchangers for water softening , nitrogen absorbers for generating oxygen by pressure swing adsorption , EDTA substitutes, molecular sieves , desiccants in dishwashers or in self-cooling beer kegs . They are also required for the large-scale production of detergents . Zeolites are among the most important catalysts in the chemical industry and are used in heat storage heating systems.
Two properties of the zeolites are used in the applications:
- The ion-exchange , ie the ability of zeolites to share their free cations against others. The largest application in terms of quantity is water softening in detergents. Another interesting application is the removal of heavy metals (including radioactive ones) from wastewater. Some zeolites show a strong affinity for certain ions (for example cesium and strontium ), the zeolite HEU was used by the BNFL ( British Nuclear Fuels ) to remove radioactive cesium from radioactive waste water and to exchange it with sodium cations. During the nuclear disaster in Fukushima , attempts were made to use zeolites to bind radioactive isotopes of cesium and strontium from the contaminated wastewater leaking into the sea .
- The adsorption capacity , i.e. the storage of neutral compounds in the micropores of the crystal structure. In adsorption , the adsorption process as such can be used, for example in the exothermic drying of gases, the most important application, for example during the drying process in dishwashers ; also in the separation of organic molecules according to size. Alternatively, the high heat of adsorption, which occurs in particular during the adsorption of water, is used. The strong driving force of adsorption is exploited when zeolites are used as energy storage , such as with self-cooling beer kegs .
Like adsorption, catalysis also takes place in the pores of the zeolite. Either the zeolite itself acts as an acidic catalyst, or the introduced metal particles are the actual active centers. An example in industry is its use as a heterogeneous catalyst for the catalytic cracking of hydrocarbons, since zeolites often have strongly acidic centers. They are also often used as bifunctional catalysts with a further metal component for various reactions .
Recently, it has also been possible to synthesize nanoscale zeolites, i.e. zeolite materials with particle diameters below 100 nanometers , which are distinguished from conventional zeolites by significantly improved transport properties. These improved properties are of outstanding importance in catalysis and in adsorption processes in which zeolites are used.
Due to their large inner surface, zeolites can be used as sorbents in sorption pumps in addition to activated carbon .
In 2000, a dietary supplement based on ground zeolite came onto the German market (trade name: Megamin ). Although there is no drug approval or has not been applied for, an alleged effect against all possible diseases such as cancer, schizophrenia or infections is advertised. There are now several suppliers who offer zeolite powder as a dietary supplement. Among other things, claims are made to have a purifying or detoxifying effect. There is no evidence for any of these claims.
For some time now, zeolites have also been used in the manufacture of low-temperature asphalt .
To stop bleeding, zeolites have been used for some time in appropriate wound compresses or sponges to help blood clot .
A use as a mobile heat storage facility is currently being researched in a test facility in Hamm .
literature
- Lotharuppe: Zeolites - properties and technical applications. In: Chemistry in Our Time. 20, 1986, p. 117, doi: 10.1002 / ciuz.19860200404 .
- AF Cronstedt: Om en obekant bärg art, som kallas Zeolites (English translation: On an Unknown Mineral-Species called Zeolites ). Akad. Handl. Stockholm 18, 1756, pp. 120-123.
Web links
- Mineral Atlas: Zeolites (Wiki)
- Zeolites in Catalysis , Chemgapedia
- Database of zeolite structures of the IZA-SC (Structure Commission of the International Zeolite Association)
- Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs
- Cooling devices with zeolite and water (BINE information service)
- Heating with a zeolite heater (BINE information service)
Individual evidence
- ^ A b c d e Moore, Elaine A. and Smart, Lesley E .: Solid state chemistry: an introduction . 4th edition. CRC Press, 2012, ISBN 978-1-4398-4790-9 , pp. 285 .
- ↑ a b M. Binnewies et alii: Allgemeine und Anorganische Chemie . 2nd Edition. Spectrum, 2010, ISBN 3-8274-2533-6 , pp. 450 f .
- ↑ Stiftung Warentest: Zeolite dishwasher - washed well 1000 times
- ↑ press release
- ↑ Bernd Müller: Not just clean, but dry! , Physik Journal, Volume 16, June 2017, pages 48 and 49
- ↑ Zeolite as heat storage , Deutschlandfunk, 2006
- ↑ Zeolite: Effect on detoxification questionable at www.medizin-transparent.at , accessed on April 3, 2017.
- ↑ aspha-min: Zeolite as an additive for the production of low-temperature asphalt
- ↑ M. Eryilmaz, T. Ozer, O. Menteş, N. Torer, M. Durusu, A. Günal, AI Uzar: Is the zeolite hemostatic agent beneficial in reducing blood loss during arterial injury? In: Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery: TJTES. Volume 15, Number 1, January 2009, pp. 7-11, PMID 19130332 .
- ↑ Mobile sorption storage at the Hamm waste incineration plant ( Memento from December 21, 2015 in the Internet Archive ). Hamm waste incineration plant. Retrieved July 25, 2013.
- ↑ Mobile sorption storage for the use of industrial waste heat ( Memento from August 30, 2013 in the Internet Archive ). Website of the Bavarian Center for Applied Energy Research. Retrieved July 25, 2013.