Zeolite Y
Zeolite Y is a technically important, artificially produced crystalline substance from the group of zeolites . Among the minerals it has its counterpart in faujasite . The most important application of zeolite Y is as a catalyst in fluid catalytic cracking (FCC process) in the refinery for the production of low-boiling fractions (gasoline and diesel) from residues of petroleum distillation.
history
After the synthesis of Zeolite A and Zeolite X with a Si / Al ratio close to 1 in 1950 by Robert Milton at Linde Air Products Division of Union Carbide , it was discovered that synthetic zeolites were susceptible to acids, water or steam due to dealumination. Mordenite , with a Si / Al ratio of 5, is less susceptible. In 1954, while trying to make synthetic zeolites with a higher Si / Al ratio, Donald Breck , a colleague of Milton's at Union Carbide, succeeded in synthesizing zeolite Y. Breck received a patent for the production of crystalline zeolite Y in 1964. Zeolite Y showed, as expected, a higher stability. Uytterhoeven et al. first determined the proton content of HY, the acidic form of zeolite Y , in 1965. In 1967, George Kerr and his colleagues at Mobil and WR Grace and Company showed that an ultra-stable form of zeolite Y (USY) could be produced by heating in an "inert static atmosphere" could be produced. In the same year, Benesi published the first thermogravimetric curves for the thermal decomposition of the ammonium form of zeolite Y.
construction
Crystal structure
The crystal structure of zeolite Y corresponds to that of faujasite. The faujasite frame consists of sodalite cages , which are connected to each other via hexagonal prisms. The pores that are formed by the composite of these secondary structures are arranged perpendicular to one another. The largest pore, which is formed by a ring with 12 units, has a diameter of 7.4 Å and is therefore relatively large compared to other zeolites. The interior of the large cavern has a diameter of 12 Å and is surrounded by 10 sodalite cages. The cell unit is cubic with a length of 24.7 Å, whereby this length can vary between 24.2 Å and 25.1 Å depending on the Si / Al ratio, type and concentration of the countercations and degree of hydration. The pore formed by the hexagonal prism has a diameter of 2.5 Å and is therefore only about a third as large as the large pore.
Sodalite cages and hexagonal prisms (secondary structures) form unit cells (tertiary structures). One unit cell contains 18 sodalite cages and 16 hexagonal prisms. Such a unit cell has the composition Na 58 Al 58 Si 134 O 125 * 240 H 2 O.
Zeolite Y of the Na form contains 52 sodium ions per unit cell, 16 of which are within the hexagonal prisms and the sodalite cages. The sodium ions in the hexagonal prisms are coordinated with 6 oxygen ions from the zeolite framework. In these prisms there is no more space for water molecules, so that the sodium ions coordinate with the oxygen ions of the zeolite framework. In the sodalite cages, they are coordinated with 3 oxygen ions from the zeolite framework and two water molecules. The remaining 36 sodium ions reside in the super cages and are fully hydrated with 6.5 molecules of water. These sodium ions are mobile and behave like in an aqueous solution.
The Si / Al ratio is 2.43 and the void volume is 48%.
Faujasite materials are characterized by a large surface and a narrow pore distribution in the range from 0.9 to 1.2 nm, as well as by a high thermal resistance. Zeolite Y decomposes above 793 ° C.
Aluminum content and aluminum distribution
The framework of zeolites consists of silicon and aluminum atoms, which are connected to one another via oxygen atoms. With "normal" zeolite Y the Si / Al ratio is approx. 2.5. However, it is possible to replace aluminum atoms with silicon atoms. This can be done through targeted dealumination . After dealumination, a distinction is made between grille aluminum and aluminum outside the grille. In most cases, the aluminum outside the lattice remains in the cavities of the zeolite. As a result of the dealumination, the unit cell shrinks and the zeolite becomes more stable.
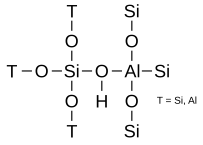
While two silicon atoms may be connected next to each other via an oxygen, there must be a silicon atom next to each aluminum atom. Two neighboring aluminum atoms are not allowed. In dehydrated zeolites of the H-form, the hydroxyl group between silicon and aluminum forms the Brönsted acid center:
Immediately after the synthesis there is a sodium ion as the counter cation at this point, after the ion exchange the corresponding ion, usually ammonium or lanthanum .
Acid centers
Each coordinated aluminum atom within the lattice framework has a negative charge with a counter cation at certain positions outside the lattice framework. In the dehydrated form, the counter cations consist of protons (Brönsted acid centers) at preferred positions that form two different acidic hydroxyl groups:
- Hydroxy groups in the super cage. They are strongly acidic and directly accessible to adsorbates.
- Hydroxy groups in the sodalite cages. They are less acidic, but mobile enough to interact with adsorbates in the supercages.
The Bronstedsaur centers are not all of the same strength. This depends on the aluminum content. If the Si / Al ratio is greater than 4.5, all centers have the same thickness. If the aluminum content is higher (Si / Al value lower than 4.5) there are centers of weak and medium activity. This is noticeable in the catalytic properties of the zeolite. If two aluminum atoms are in the immediate vicinity of a silicon atom, the strength of the Brönsted acid centers is reduced. For this reason, the strength of the individual Bronsted acid centers decreases with increasing aluminum content.
The aluminum content also has an effect on the oscillation frequency of the OH expansion of the Brönsted acid centers, as the IR spectrum shows. These different centers are assigned to different crystallographic positions. In the first case the proton points towards the large cavern, in the second case it is inside the sodalite cage (see picture with positions of the oxygen atoms).
to form
Depending on the counterion on the aluminum, a distinction is made between "forms":
- NaY is the original form with sodium.
- HY is the sodium-free, acidic form. This name was used in 1969 by Kerr et al. introduced.
- NH4Y is the form with ammonium as the counterion. Calcination results in HY.
Furthermore, one differentiates according to the stability:
- USY for ultra stable zeolite Y ( ultra stable ), which is obtained by treatment with steam at high temperatures, in which a dealumination of the lattice takes place.
- VUSY for very ultrastable
matrix
The zeolite crystals are too small to be used directly as such in the FCC unit . In order to be able to separate them from the reaction mixture, particles with a minimum size are required. The zeolite crystals are therefore embedded in a matrix. This matrix takes the form of a microsphere, so-called "microspheres" with a size of approx. 70 µm. These can be separated by gravity in the separator of the FCC unit.
The matrix can be catalytically inert or active.
Catalytically inert matrices
There are two types of catalytically inert matrices:
- Derived from silica gel
- Derived from clay
Silica gel-derived matrices
A silica hydrosol (a colloidal suspension ) is made by acidifying a sodium silicate solution to pH 3 . The zeolite crystals and fine clay are stirred in. The mixture is then spray-dried and the resulting microspheres are further processed by ion exchange and calcination. The end product contains the active zeolite in a matrix of amorphous silica gel and clay. Such a catalyst is inexpensive and resistant to abrasion, but the matrix has only small pores and a small internal surface area, which makes no significant contribution to the cracking reaction.
Alumina-derived matrices
Such a matrix is made by crystallization in situ , i.e. H. within the matrix itself. This technology was developed by Engelhard Corporation . For this purpose, ground, hydrated kaolin is calcined at approx. 1000 ° C, which converts it into spinel . The calcined material is mixed with more hydrated kaolin, a small amount of sodium silicate, and zeolite seeds. The resulting mixture is spray dried and the resulting microspheres calcined at 732 ° C to convert the hydrated kaolin to metakaolin (see also kaolinite ). The spinel and metakaolin are held together within the microsphere by the sodium silicate as a binding agent. The calcined microspheres are then treated in a mixture of sodium silicate and sodium hydroxide solution, with part of the matrix (the metakaolin) crystallizing to form zeolite Y.
Catalytically active matrices
There are two types of catalytically active matrices:
- Alumina sol-derived matrices
- Amorphous silicon dioxide / aluminum oxide matrices
Alumina sol-derived matrices
The catalytically active matrix consists of amorphous aluminum oxide. The zeolite is ion-exchanged and modified before being added to the matrix. Pseudoboemite is first peptized with an acid and then mixed with clay and the appropriate Y zeolite. The spray-dried product has a catalytically active matrix with a high specific surface area, which increases the octane number of the product and is effective in cracking residue.
Amorphous SiO 2 / Al 2 O 3 matrices
These matrices can be made from a SiO 2 / Al 2 O 3 hydrogel or hydrosol. Such a matrix increases the catalytic activity and the octane number.
synthesis
Zeolite Y is produced industrially by crystallization, which is generally followed by an ion exchange with subsequent calcination at a mild temperature and a second ion exchange with a second calcination at a higher temperature. There are several manufacturing processes, but they can be generalized according to the following scheme.
An aluminum (sodium aluminate) and a silicon source (sodium silicate, water glass) are used for crystallization. But it can also be used other substances that contain both elements, such. B. kaolin . Naturally occurring kaolinite is the preferred source because of its low cost. However, this cannot be used as such, but must first be calcined at a high temperature (700 ° C to 900 ° C) in order to make it chemically active by converting it to metakaolin.
In the first ion exchange, the sodium content is reduced from approx. 9% to approx. 2%. The first calcination "mobilizes" the remaining sodium ions and makes them accessible for a second ion exchange. In the second calcination, the ammonium ions are removed as ammonia, leaving acidic centers behind.
Crystallization
Like other zeolites, zeolite Y is produced by crystallization from aluminum (e.g. sodium aluminate ) and silicon- containing substances (e.g. sodium silicate or water glass ). Other aluminosilicates such as kaolin can also serve as sources of aluminum and silicon. The starting materials are in an alkaline medium, e.g. B. sodium hydroxide solution , dissolved and crystallized at temperatures between 70 ° C and 300 ° C (mostly around 100 ° C). This is not a crystallization in the classical sense, in which the solubility limit of a dissolved substance is exceeded so that it changes into the solid state, but rather a chemical reaction in which the individual components (aluminates and silicates) are in shape a crystal lattice are joined together.
Ion exchange
Once synthesized, the zeolite is in its sodium form; H. the counterions of the aluminum ions consist of sodium cations. It is converted into the ammonium form by ion exchange with aqueous solutions of ammonium salts (e.g. ammonium nitrate or ammonium sulfate ) . The sodium cations are replaced by ammonium cations and get into the mother liquor . The ion exchange can be carried out, for example, by stirring in containers with the addition of the ammonium salt solution.
The sodium cations in the super cages can easily be exchanged as they are hydrated and mobile. The sodium ions in the hexagonal prisms, on the other hand, are coordinated with the oxygen ions of the zeolite structure and are therefore difficult to access. They cannot be ion exchanged easily. These sodium ions are “mobilized” by calcination at a relatively mild temperature (600 ° C) and can be exchanged in a subsequent, second ion exchange. The sodium ions migrate outside the hexagonal prisms during the calcination. Aluminum migrates as a counterion for the sodium, which leads to a dealumination of the framework, which creates mesopores. The second ion exchange can reduce the sodium content to around 1000 ppm, which is important for the stability of the end product. Philip Maher and Carl McDaniel from WR Grace and Company received a patent in 1975 for improved ion exchange on crystalline zeolites by intermediate calcination.
The ion exchange is also influenced by the pH value. The degree of exchange of sodium is higher, the lower (more acidic) the pH is. The problem here is the destruction of the crystal structure if the pH values are too low. It was found that there is no loss of crystallinity between pH 3.0 and 4.8 during the first ion exchange. It is therefore advantageous to carry out the first ion exchange at a pH of 3.0. In the second ion exchange, however, a decrease in crystallinity to 70% was already found at a pH value of 3.0. The second ion exchange should therefore be carried out at a higher pH value of 4.5.
In order to stabilize the zeolite structure, part of the sodium is usually replaced by rare earth metals , mostly lanthanum , and sometimes also cerium , also through ion exchange. The replacement of the monovalent sodium ion by divalent or trivalent cations leads to an increase in the lattice energy and thereby increases the structural stability. At 25 ° C, the hydrated lanthanum ions are too large to diffuse from the supercages into the hexagonal prisms. The hexagonal prisms have a free diameter of 0.25 nm, while the hydrated La 3+ ions are larger at 0.396 nm (0.79 nm loud). In order for lanthanum to diffuse into the small sodalite cages through the hexagonal prisms, the hydrate shell must be mobilized by increasing the temperature to 82 ° C (50 ° C to 90 ° C according to).
A solid phase ion exchange is also possible, but rather a rarity and of no technical significance. For this purpose, the dry salt is brought into contact with the zeolite at a high temperature. This method can be used to exchange H-form zeolites with lanthanum, e.g. B. with LaCl 3 . The salt molecules diffuse to the exchange points.
Calcination
The ammonium ion is removed by subsequent calcination and the zeolite is converted into its acidic form. In what is known as ultra stabilization, the zeolite is treated with steam at 700 ° C to 800 ° C. The zeolite loses aluminum and the specific surface area decreases.
The countercations, especially sodium or lanthanum, lose their hydration shells as a result of the calcination. This allows them to diffuse through the hexagonal prisms into the smaller sodalite cages. For example, lanthanum ions can diffuse into the sodalite cages from temperatures around 100 ° C. At temperatures higher than 200 ° C in the presence of lanthanum, a hydroxyl group and a proton are formed on each lanthanum ion. The acid thus formed leads to dealumination of the lattice.
Dealumination
The atomic ratio Si / Al in zeolites is an important parameter that defines properties such as maximum ion exchange capacity, thermal and hydrothermal stability, hydrophobicity , and the number and strength of the Brönsted acidic centers . Zeolites with a low aluminum content tend to be more stable, especially when used as FCC catalysts. Since the synthesis of zeolite Y is only possible in a narrow Si / Al range without great effort, it cannot be produced directly by crystallization with an Si / Al ratio greater than 2.5. The Si / Al ratio can, however, be increased by later removing aluminum from the grid. This leads to lattice defects, which can be partially reoccupied by installing other elements, in particular silicon. As a result of the dealumination, the size of the cell unit decreases slightly, since the SiO bonds are shorter than the Al-O bonds.
There are different dealumination procedures, the most important of which are the following:
- Hydrothermal treatment (with hot steam)
- Acid treatment
- Treatment with gaseous hallids or halogens
- Complexation with chelating agents such as EDTA
The elimination of the AlO 2 groups reduces the number of (sodium) counter cations. This also reduces the maximum possible load with rare earth metals.
Dealumination also leads to the formation of mesopores, which improves the catalytic properties during cracking.
The aluminum atoms that are removed from the zeolite lattice form aluminum species outside the lattice, which have been implicated in the formation of octahedral aluminum. It has been suggested that these aluminum species form a separate phase outside of the zeolite crystal. Alternatively, it has been suggested that they exist as isolated cations within the zeolite cages and compensate for the electrical charge. Tetrahedrally coordinated aluminum can also be located outside the grid . The presence of aluminum species outside the lattice leads to better hydrothermal stability, increased Lewis acidity and increased catalytic activity in reactions that require strong Brönsted acids, such as e.g. B. cracking.
Hydrothermal dealumination
The hydrothermal treatment is the technically preferred variant for removing aluminum from the framework and increasing the stability of the zeolite. During dealumination by calcination with steam at high temperatures, some of the aluminum in the grid migrates to places outside the grid:
It is essentially a high-temperature hydrolysis of the Si-O-Al bonds with the formation of aluminum species outside the lattice structure. The aluminum hydroxide formed can react as an amphoteric substance with the acidic centers on the zeolite and is accessible through ion exchange:
Under these conditions, a restructuring of the zeolite framework takes place while eliminating the defects that have arisen. These are occupied by advancing silicon:
The result is an ultra-stable Y zeolite (USY) with a higher silicon content, which is characterized by high thermal and hydrothermal stability, a smaller cell unit and reduced ion exchange capacity. Sodium-free zeolite in the ammonium form is usually used for this type of dealumination. The calcination is carried out at temperatures between 500 ° C and 870 ° C.
Desilification
Under desilication means the removal of silicon atoms from the zeolite framework. After the intensive investigation of the dealumination of zeolites, interest in desilification is increasing. This allows the mesoporosity to be increased post-synthetically. This improves the diffusion properties of the zeolite. In general, zeolites are desilicated by treatment with sodium hydroxide solution .
properties
Mobility of ions
In the hydrated form, the ions and water molecules in the super cage are very mobile, which allows both ion exchange and reversible dehydration and sorption.
stability
H-form zeolite Y, which is obtained by calcining the ammonium form, is very unstable in the presence of water. This instability was attributed to the intrinsic acidity of this form when lattice hydrogen ions form H 3 O + ions with intergranular, “liquid” water . This leads to a dealumination of the lattice similar to that produced by treatment with acid.
The thermal stability of the zeolite lattice depends essentially on the counter cation on the aluminum, its distribution in the lattice and the degree of ion exchange. Ion exchange with multivalent cations, especially those of rare earth metals, increase the thermal stability of zeolite Y (see synthesis and ion exchange).
Ultra stable zeolite (USY)
To be suitable as a catalyst in the FCC process, the material must be resistant to temperatures around 700 to 950 ° C in the presence of water vapor. Although the provision of sodium-free Y-zeolite (HY) represented a significant advance, it was only stable up to temperatures around 500 ° C. It was noted that in the presence of humidity even at room temperature, the structure of HY was damaged. The stability could be increased by exchanging ions with polyvalent cations. In parallel, other methods of increasing stability have been developed. Zeolites with a low aluminum content are usually more thermally and chemically stable. Since the synthesis of zeolite Y is only successful in a certain range with a low Si / Al ratio, methods for the subsequent removal of aluminum were worked out (see dealumination ).
Barrer and Makki reported for the first time in 1964 on a post-synthetic method to dealuminate zeolites ( clinoptilolite ) in a targeted manner by treatment with a mineral acid. In 1967 McDaniel and Maher reported for the first time on the production of a stable Y zeolite, which they called "ultra stable". This material was prepared by calcining the ammonium form (NH 4 Y) at above 500 ° C., which initiated dealumination. Kerr showed in 1967 that the water formed during the dehydroxylation of HY plays a crucial role in the ultra-stabilization. When HY was calcined at 650 ° C to 750 ° C while purging with an inert gas, a zeolite with low stability resulted. By flushing with inert gas, the water that was formed by splitting off hydroxyl groups was removed. On the other hand, if the calcination at 700 ° C to 800 ° C without inert gas purging, i. H. in the presence of the water formed, the product (USY) was more stable. With subsequent thermal stress up to 1000 ° C it remained crystalline.
La-exchanged Y zeolites
To increase the stability and improve the catalytic properties, Y zeolites are mixed with rare earth metals, mainly lanthanum and, to a much lesser extent, cerium (see ion exchange). Above 10% by weight content of rare earth metals, the improvement in the octane number during cracking no longer increases significantly. Y Zeolites with high rare earth content are primarily used to maximize gasoline yield. Y zeolites with a lower content are mainly used to reduce coking and the formation of fission gases. With an increasing content of rare earth metals, the octane number of the product increases and the gasoline yield decreases.
La-exchanged USY has both Bronsted and Lewis acidic centers. The acidity of the Y zeolite decreases due to the ion exchange with lanthanum, because some of the protons on the surface are replaced by lanthanum. The acidity thus decreases in the order La, HY> USY> La, H-USY>.
The resistance to treatment with steam at high temperatures (540 ° C) increases due to the ion exchange with lanthanum. This test is carried out to simulate the conditions in the FCC unit. Treatment with hot steam can restructure the lattice from the zeolite, which can lead to the separation of water and dealumination. This causes the unit cell to shrink. The presence of the relatively large La ions (r = 1.15 A) in the zeolite cages prevents the cell unit from shrinking and the formation of new cationic, aluminum-containing species. The zeolite becomes more stable as the La content increases.
The thermal treatment of La-exchanged zeolite Y (RE, HY) creates additional acidic centers due to the hydrolysis of partially hydrated lanthanum ions. RE, HY calcined at 480 ° C has only Bronsted acid centers, while calcination at higher temperatures decreases the Bronsted acidity by dehydroxylation with the formation of Lewis acid centers. The hydrothermal treatment leads to partial hydrolysis of the connection between lanthanum and zeolite and a partial dealumination of the framework with the associated decrease in overall acidity and unit cell size.
Xu et al. were able to demonstrate through images with the scanning transmission electron microscope that in partially exchanged LaY (still containing Na) the lanthanum ions were mainly present as individual cations, but that lanthanum pairs are also present in a ratio of 1: 4, which are presumably bridged by an oxygen atom.
Abrasion resistance
The abrasion resistance of the finished catalyst is important for several reasons:
- It can determine the amount of catalyst that has to be constantly replaced in the FCC unit.
- It can influence the fluidizing properties of the catalyst in the FCC unit as the size distribution of the particles changes.
- It determines the amount of fine particles that are emitted from the FCC unit.
The abrasion resistance is determined by the matrix and the zeolite content. In general, the abrasion resistance decreases with increasing zeolite content, which can become critical at contents of over 35% Zeloth.
Pore volume and pore distribution
The porosity of the catalyst is largely determined by its composition, manufacturing method and hydrothermal treatment. It is an important variable that influences the catalytic properties of the catalyst. With predominantly small pores (<100 Å) there is a risk of clogging due to coking . Macropores that are too large (> 200 Å) are often associated with a specific surface that is too low, which reduces the catalytic activity and the abrasion resistance. An ideal catalyst contains a balanced distribution of micro and macro pores. While the micropores ensure a sufficiently high catalytic activity, the macropores allow a sufficiently rapid diffusion of the reactants through the catalyst. The use of surfactants as templates enables the formation of mesostructured pores in the range from 2 to 50 nm. For example, the zeolite is first treated with citric acid and then with cetyltrimethylammonium bromide (CTAB) and sodium hydroxide solution. Enclosed CTAB is first coked by calcination at 550 ° C. and then burned by introducing air at this temperature. The remaining voids represent pores with relatively defined dimensions, through which larger molecules can more easily get into the zeolite interior and the products formed can diffuse out more quickly. This achieves a better selectivity of the cleavage reaction.
use
Main application
Zeolite Y is mainly used as a cracking catalyst in the FCC process to convert residues into diesel or gasoline in the refinery. The quantities produced for this purpose are in the range of several hundred thousand tons per year. Zeolite Y has replaced zeolite X as a catalyst because it has a higher stability due to its higher Si / Al ratio and is also more active. It is also used in the hydrocracking process as a carrier for platinum / palladium in order to increase the aromatic content.
Other significant uses
- Nickel containing zeolite Y can be used as a catalyst in hydrogenation reactions, e.g. B. in the hydrogenation of carbon monoxide in the Fischer-Tropsch synthesis , in the hydrogenation of unsaturated hydrocarbons or in the hydrogenolysis of saturated compounds.
Less significant applications
- Zeolite Y can also be used as a catalyst for the production of N-methylaniline .
- Cobalt-containing zeolite Y can also be used for hydrogenation reactions. Only zeolite Y is active here, which is impregnated with Co 2 (CO) 8 (see cobalt carbonyl hydride ). Ion-exchanged zeolite Y, e.g. B. from cobalt (II) nitrate , is not hydrogenation-active, since the cobalt is in this case in the sodalite cages and does not migrate out of them.
Individual evidence
- ↑ Masters, A .; Maschmeyer, Th .; Zeolites - From curiosity to cornerstone . In: Microporous and Mesoporous Materials 142 (2011) 423-438
- ↑ US Patent 3,130,007 Cyrstalline Zeolite Y
- ↑ Uytterhoeven, JB, Christner, LG, Hall, WK; J. Phys. Chem. (1965) 69 , 2117
- ↑ a b c Kerr, GT, Hydrogen Zeolite Y, Ultrastable Zeolite Y, and Aluminum-Deficient Zeolites , Advances in Chemistry Series, 121 / 219-229, 1973
- ↑ a b Kerr, GT; The Intercrystalline Rearrangement of Constitutive Water in Hydrogen Zeolite Y , J. Phys. Chem. 71 (1967) 4155-4156
- ↑ Benesi, HA, J. Catal. (1967) 8 , 368
- ↑ a b Sierka, M., Eichler, U., Datka, J., Sauer, J .; Heterogeinity of Brönsted Acidic Sites in Faujasite Type Zeolites due to Aluminum Content and Framework Structure , J. Phys. Chem. B (1998), 102, 6397-6404
- ^ A b The Chemical Engineering Zeolite Page
- ↑ Kaduk, A .; Crystallopgraphy Reviews, 11 (1) (2005) 1-19
- ^ Breck, DW, Zeolite Molecular Sieves , 1974, Wiley & Sons
- ^ Marinsky, JA, Ion Exchange , Volume 2, 169, Dekker Inc.
- ↑ a b "Synthesis and catalytic characterization of zeolite Y with different crystal sizes" , dissertation by Christine Berger
- ↑ Karami, D .; Rohani, S .; Synthesis of pure zeolite Y using soluble silicate, a two-level factorial experimental design , Chem. Eng. and Proc. 28 (2009) 1288-1292.
- ↑ Son, JR, DeCanio, SJ, Lunsford, JH, O'Donnell, DJ; Determination of framework aluminum content in dealuminated Y-type zeolites: a comparison based on unit cell size and wavenumbers of IR bands , Zeolites (1986) Vol. 6, 225-227
- ↑ a b c d e f g h i j Scherzer, J., Octane-Enhancing, Zeolitic FCC-Catalysts: Scientific and Technical Aspects , Catal. Rev. Sci. Closely. 1989, 31 (3): 215-354
- ↑ Kerr, GT, Cattanach, J., Wu, EL; J. Catal. (1969) 13 , 114.
- ↑ a b Scherzer, J .: Dealuminated Faujasite-Type Structures with SiO 2 / Al 2 O 3 Ratios over 100 , Journal of Catalysis, 54 , (1978), 285-288
- ↑ Haden, WL, et al .; US Patent 3,663,165 (1972)
- ↑ Brown, SM et al .; US Patent 4,493,902 (1985)
- ↑ Stockwell, D. et al .; US Patent 6,656,347 (2003)
- ↑ Wang, JQ et al .; New hydrothermal route for the synthesis of high purity nanoparticles of zeolite Y from kaolin and quartz , Microporous and Mesoporous Materials 232 (2016) 76-85
- ↑ a b U.S. Patent Re. 28,629 Ion Exchange of Crystalline Zeolites
- ↑ a b c Sato, K., et al., Structural changes of Y zeolites during ion exchange treatment: effects of Si / Al ratio of the starting NaY , Microporous and Mesoporous Materials, 59, (2003), pp. 133-146
- ↑ a b Shiralkar, VP, Kulkarni, SB, Thermal and structural properties of rare earth exchanged zeolites , Journal of Thermal Analysis , Vol. 25, (1982), pp. 399-407
- ↑ a b Lee, EFT, Rees, VC, Effect of calcination on location and valency of lanthanum ions in zeolite Y , Zeolites, 1987, Vol. 7, pp. 143-147
- ↑ a b Schuessler, F. et al .: Nature and Location of Cationic Lanthanum Species in High Alumina Containing Faujasite Type Zeolites, J. Phys. Chem. C , 2011, 115, 21763-21776
- ^ Howard, S., The ion exchange properties of zeolites, III. Rare earth ion exchange of synthetic faujasites , Journal of Colloid and Interface Science, Vol. 28, No. October 2, 1968
- ^ Sulikowski, B. et al .: Solid-state ion exchange in zeolites: Part 8: Interaction of Lanthanum (III) chloride with zeolites under anhydrous conditions , Zeolites (19), 1997, 395-403
- ↑ a b Beyer, HK: Dealumination Techniques for Zeolites, Molecular Sieves - Post-Synthesis Modification I , Springer Verlag, 2002, pp. 204-255
- ↑ a b c d e van Bekkum, H., et al., Introduction to Zeolite Science and Practice, 2nd Completely Revised and Expanded Edition , Elsevier 2001
- ↑ Sievers, C., et al., Stability of Zeolites in Hot Liquid Water , J. Phys. Chem. C, 2010, 114, 19582-19595
- ↑ Kerr, GT, J. Phys. Chem. 71: 4155 (1967)
- ↑ a b Silaghi, M.-C., Chizallet, C., Raybaud, P .; Challenges on molecular aspects of dealumination and desilication of zeolites , Microporous and Mesoporous Materials, 191 (2014) 82-96
- ↑ US Patent 3,506,400 (1970)
- ↑ Bremer, H., Morke, W., Schodel, R., Vogt, F .; Adv. Chem. Ser., 121 , 249 (1973)
- ↑ Barrer, R M., Makki, MB: Molecular Sieve Sorbents from Clinoptilolite . In: Canadian Journal of Chemistry . 42 (6), 1964, pp. 1481-1487, doi : 10.1139 / v64-223 .
- ↑ McDaniel, CV, Maher, PK; Molecular Sieves, in: rM Barrer (Ed.), Society of Chemical Industry, London, pp. 186-195
- ↑ Scherzer, J., Bass, JL: Ion Exchanged Ultrastable Y Zeolites, I. Formation and Structural Characterization of Lanthanum-Hydrogen Exchanged Zeolites, Journal of Catalysis , 46 (1977), 100-108
- ↑ Xu, P. et al .: Imaging individual lanthanum atoms in zeolite Y by scanning electron microscopy: Evidence of lanthanum pair sites, Microporous and Mesoporous Materials , 213 (2015) 95-99
- ↑ García-Marínez, J., Li, K., Krishnaiah, G .; A mesostructured Y zeolite as a superior FCC catalyst - from lab to refinery , Chem. Commun. 2012, 48 , 11841-11843
- ↑ US: Patent 20110118107
- ↑ a b Coughlan, C., Ó Domhnaill, C .; Catalysis on cobalt-containing sodium zeolite Y . In: Topics in Catalysis , 1 (1994), 163-167
literature
- Subhash Bhatia: Zeolite Catalysis. Principles and Applications . CRC Press Inc., Boca Raton FL 1990, ISBN 0-8493-5628-8 .
- F. Ramoa Ribeiro et al. a. (Ed.): Zeolites. Science and Technology . Proceedings of the NATO Advanced Study Institute on Zeolites, Science and Technology, Alcabideche, Portugal, May 1-12, 1983. Martinus Nijhoff Publishers et al. a., The Hague et al. a. 1984, ISBN 90-247-2935-1 ( NATO ASI series : E: Applied sciences 80).
Web links
- “Synthesis and catalytic characterization of zeolite Y with different crystal sizes” , dissertation by Christine Berger
- The Chemical Engineering Zeolite Page
- James A. Kaduk, Johan Faber: Crystal Structure of Zeolite Y as Function of Ion Exchange (PDF; 3.1 MB). The Rigaku Journal, Vol. 12, No. 2, 1995









![{\ displaystyle \ mathrm {Al (OH) _ {3} + H ^ {+} [Y] \ longrightarrow Al (OH) _ {2} ^ {+} [Y] + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5aced712fdc29fdc436f4b1aea6cb7f249634f13)


