Sodalite
| Sodalite | |
|---|---|
| Sodalite with some pyrite from the lapis lazuli deposit Ladjuar Medam, Sar-e Sang , Afghanistan | |
| General and classification | |
| chemical formula | Na 8 [Cl 2 | (AlSiO 4 ) 6 ] |
|
Mineral class (and possibly department) |
Silicates and Germanates |
|
System no. to Strunz and to Dana |
9.FB.10 ( 8th edition : VIII / F.07) 76.02.03.01 |
| Similar minerals | Lapis lazuli , leucite , analcim , nosean , haüyn |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic-hexakistrahedral; 4 3 m |
| Space group | P 4 3 n (No. 218) |
| Lattice parameters | a = 8.88 Å |
| Formula units | Z = 1 |
| Twinning | to {111}; pseudo-hexagonal prisms according to [111] |
| Physical Properties | |
| Mohs hardness | 5.5 to 6 |
| Density (g / cm 3 ) | measured: 2.27 to 2.33; calculated: 2.31 |
| Cleavage | indistinct after {110} |
| Break ; Tenacity | uneven to scalloped |
| colour | colorless, white, yellowish, greenish, light to dark blue, reddish; colorless to gray in thin layers |
| Line color | White |
| transparency | transparent to translucent |
| shine | Glass gloss, fat gloss |
| Crystal optics | |
| Refractive index | n = 1.483 to 1.487 |
| Birefringence | none, as isotropic |
| Other properties | |
| Special features | orange-red fluorescence |
Sodalite is a rarely occurring minerals from the mineral class of "silicates and germanates" with the chemical composition Na 8 [Cl 2 | (AlSiO 4 ) 6 ] and is therefore chemically seen a sodium - aluminosilicate with additional chlorine ions .
Sodalite crystallizes in the cubic crystal system and develops mostly granular to massive mineral aggregates with a size of up to over a meter, more rarely small, millimeter to centimeter-sized crystals in mostly gray-blue to dark blue color. Depending on foreign admixtures or inclusions, sodalite can also take on a white, yellow or purple to pink (hackmannite) color. Colorless crystals are also known.
Sodalite belongs to the foids and together with bicchulite , danalite , genthelvin , haüyn , helvin , kamaishilite , lazurite , nosean , tsaregorodtsevit and tugtupite, a mineral group named after him .
Etymology and history
The name sodalite is a loan word made up of the Latin sodium for sodium and the Greek λίθος lithos for stone and refers to its high sodium content.
Sodalite was first found in the Ilímaussaq massif in the province of Kitaa (West Greenland) and described by Thomas Thomson in 1812 .
In 1930, Linus Pauling published a first proposal for the structure of sodalite, which Jürgen Löns and H. Schulz confirmed in their crystallographic work in 1967 .
classification
Already in the outdated 8th edition of the mineral classification according to Strunz , sodalite belonged to the mineral class of "silicates and germanates" and there to the department of "tectosilicates (tectosilicates) with zeolites ", where together with Nosean he created the "sodalite-nosean series" with the system no. VIII / F.07 and the other members Haüyn Lasurit and Tugtupit .
In the last revised and updated Lapis mineral directory by Stefan Weiß in 2018 , which, out of consideration for private collectors and institutional collections, is still based on this old form of Karl Hugo Strunz's system , the mineral was given the system and mineral number. VIII / J.11-10 . In the “lapis system”, this also corresponds to the “framework silicates” department, where sodalite forms an independent but unnamed group together with bicchulite , Haüyn, hydrosodalite , kamaishilite , lazurite, nosean, tsaregorodtsevit , tugtupit and vladimirivanovite .
The 9th edition of Strunz's mineral systematics , which has been valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, classifies sodalite in the more finely subdivided section of “tectosilicates without zeolitic H 2 O”. This is also further subdivided according to the possible presence of additional anions , so that the mineral can be found according to its composition in the sub-section “Tectosilicates with additional anions”, where together with Danalith the “Sodalith-Danalith group” with the System no. 9.FB.10 and the other members Bicchulith, Genthelvin , Haüyn, Helvin , Kamaishilith, Lasurit, Nosean, Tsaregorodtsevit and Tugtupit.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns sodalite to the class of "silicates and germanates" and there in the department of "structural silicates: Al-Si lattice". Here he is also the namesake of the " sodalite group " with the system no. 76.02.03 within the sub-section “Framework silicates: Al-Si lattices, feldspar representatives and related species”.
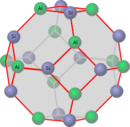
Crystal structure
Sodalite crystallizes in the cubic crystal system in the space group P 4 3 n (space group no. 218) with the lattice parameter a = 8.88 Å and one formula unit per unit cell .
The sodalite structure (Fig. 1) can be described as a cubic closest packing of rings of 6 in the direction [111]. Aluminum, silicon and oxygen atoms form the covalent structure of the lattice. Figure 2 shows the positions of the Al and Si. Between the Al and Si there is an O near the red connecting lines. The lattice carries negative charges and forms ionic bonds with sodium cations. (see aluminosilicate ) This structure requires the chemical composition Na 6 [Al 6 Si 6 O 24 ] and is colorless. Every sodalite cage of this composition has an empty space inside and can contain other substances (cations and anions or even water). These substances can be the cause of the colors of the sodalite-based minerals.
Fig. 1: Sodalite cage Fig. 2: Sodalite cage with the positions of Al and Si Fig. 3: Sodalite from 8 sodalite cages, a ninth cage is created in the center.
properties

Depending on where it was found, sodalite shows strong, orange-red fluorescence and yellow phosphorescence under long-wave and short-wave UV light .
Sodalite is easily soluble in weak to moderately strong acids such as hydrochloric acid, whereby it initially discolors and after a while dissolves with the precipitation of silica gel . The reactions, especially the loss of color, also run faster when exposed to heat. Water that is already boiling is able to remove sodium and chlorine from the sodalite.
Modifications and varieties

As Hackmanit is a sulfate-containing , violet referred to pink variety, the first time in 1991 in Quebec City was discovered (Canada) in abrasive worthy quality. A special property of the hackmanite from Sar-e-Sang is its photochromism (English tenebrescence ), probably caused by color centers . In contrast to the "normal" hackmanite, its color does not fade under sunlight, but becomes more intense. The effect is even stronger when using a UV lamp, under the influence of which the color can be increased to a strong purple within tenths of a second. In addition, there is a pink to orange-colored fluorescence . Hackmanites from other sites, on the other hand, recharge their color in the dark.
Related minerals
Lasurite (also called ultramarine) is part of the mineral mixture lapis lazuli . Sodalite with S 3 - - and S 2 - - radicals generated by the assembly ( coordination ) in the sodalite cages an intense blue color.
Nosean also has the framework structure of sodalite, but only every second cage is occupied by the divalent sulfate anion. The connection is colorless.
Education and Locations
Sodalite mostly forms in igneous rocks with a medium to low SiO 2 content such as nepheline syenites , phonolites and related rocks, but also metasomatically in calcareous rocks and marble . Accompanying minerals include aegirine , anchorite , albite , andradite , barite , calcite , cancrinite , fluorite , nepheline , microcline and sanidine .
As a rather rare mineral formation, sodalite can sometimes be abundant at various sites (sometimes even rock-forming), but overall it is not very common. So far (as of 2016) around 500 sites are known worldwide. In addition to its type locality Ilímaussaq massif, sodalite was also found in other regions of the province of Kitaa and the province of Tunu in Greenland .
Other previously known sites include Badachschan (Sar-e-Sang) and Lugar in Afghanistan; Shvanidzorskii in Armenia ; New South Wales and Tasmania in Australia; Cochabamba in Bolivia; the northeast and southeast regions of Brazil ; Shaanxi in China; Baden-Württemberg (Kaiserstuhl) and Rhineland-Palatinate (Eifel) in Germany; Auvergne-Rhône-Alpes in France; Thrace in Greece; Los Archipelago in Guinea ; Apulia , Campania and Latium in Italy; the southern region of Cameroon ; British Columbia , Ontario and Quebec in Canada; near Almaty and Aqtöbe in Kazakhstan; Chonashu (Irtashskii) in Kyrgyzstan ; Kivu in the Congo; Pokchin-san in Korea ; at Balaka and Chitipa in Malawi ; at Kidal in Mali; Meknes-Tafilalet in Morocco; Chihuahua in Mexico; in the Gobi Desert (Mongolia); Mandalay in Myanmar; Khomas in Namibia; several regions in Norway ; Burgenland and Styria in Austria; Puno in Peru; in the Azores and near Faro in Portugal; in Harghita County in Romania; some regions in Russia ; South Africa ; in the Canary Islands in Spain; several regions in Sweden ; Ticino in Switzerland ; Arusha in Tanzania; Scotland in Great Britain; Bohemia in the Czech Republic; Donetsk in Ukraine; several regions of the US ; as well as on the Amazon in Venezuela. Two sites in Zambia, near Solwezi and Lusaka.
use
Sodalite is often processed into gemstones in the form of gems and small sculptures , but also spheres or cabochons for necklaces and faceted for rings due to its often lively speckled color . Large, deep blue stones are sometimes referred to as “Royal Blue”, blue stones as “Blue Sapo”, blue stones with a few light inclusions as “Blue Tiger” and light blue stones with white inclusions as “Nuvolato”.
Sodalite is also used as a decoration in aquaristics .
Large deposits such as in Bolivia, Brazil, Zambia and Namibia are processed into floor and wall tiles or facade panels, although the Namibian deposit is currently not being mined. Bolivian material is very rarely available on the market, as mining takes place under difficult conditions. Blue King from Zambia is also no longer being mined because it is not optically attractive as a decorative stone. Only the Brazilian material, trade name Azul Bahia, has been launched on the market .
As a pigment , sodalite is of minor importance. Its related mineral lazurite and the mineral mixture lapis lazuli are preferred as pigment suppliers.
In science, synthetic sodalites, whose composition often differs from that of the mineral, serve as a model system for the zeolite group of substances . The sodalith cage is a structural component of compounds with important zeolite A , zeolite X and zeolite Y . The technical synthesis of sodalites is mostly done hydrothermally.
See also
literature
- Thomas Thomson : A chemical analysis of sodalite, a new mineral from Greenland . In: Transactions of the Royal Society of Edinburgh . tape 6 , 1812, pp. 387–395 , doi : 10.1017 / S0080456800028416 , PMC 5699400 (free full text) - (English).
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 609 .
- Petr Korbel, Milan Novák: Mineral Encyclopedia (= Villager Nature ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 268 .
- Jaroslav Bauer, Vladimír Bouška: Gemstone Guide . Verlag Werner Dausien, Hanau / Main 1993, ISBN 3-7684-2206-2 , p. 158 .
Web links
- Mineral Atlas: Sodalite (Wiki)
- Sodalite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF)(English).
- American-Mineralogist-Crystal-Structure-Database - Sodalite. In: rruff.geo.arizona.edu. (English).
- Kremer Pigments - Sodalite ( Memento from March 7, 2016 in the Internet Archive )
Individual evidence
- ↑ a b c d e Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 699 (English).
- ↑ David Barthelmy: sodalite MineralData. In: webmineral.com. Retrieved December 12, 2019 .
- ↑ a b c d e Sodalite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 70 kB ; accessed on December 12, 2019]).
- ↑ sodalite. In: mindat.org. Hudson Institute of Mineralogy, accessed December 12, 2019 .
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1816 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed December 12, 2019 .
- ^ A b Walter Schumann: Precious stones and gemstones. All kinds and varieties. 1900 unique pieces . 16th, revised edition. BLV Verlag, Munich 2014, ISBN 978-3-8354-1171-5 , pp. 190 .
- ↑ a b Sodalite: Gemmologie Mineralogie Gesteinskunde Petrologie Chemie. karrer-edelsteine.de, accessed on December 26, 2016 .
- ↑ Thomas Hainschwang, Hubert Heldner: Hackmanite, a variety of sodalite. In: free-form.ch. Free Form Artists, accessed December 12, 2019 .
- ^ Database of Luminescent Minerals - Hackmanite. In: fluomin.org. Retrieved December 12, 2019 .
- ↑ Localities for sodalite. In: mindat.org. Hudson Institute of Mineralogy, accessed December 12, 2019 .
- ↑ List of locations for sodalite in the Mineralienatlas and Mindat , accessed on December 12, 2019.