Labetalol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without stereochemistry (mixture of four stereoisomers) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Labetalol | ||||||||||||||||||
| other names |
(±) -2-Hydroxy-5- {1-hydroxy-2 - [(1-methyl-3-phenylpropyl) amino] ethyl} benzamide |
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Labetalol is a chemical compound from the group of salicylic acid - derivatives and benzamides . It is used as a medicine to treat hypertension ( blood pressure lowering agent ). It works as both an alpha and beta blocker . The compound was patented by Allen & Hanburys (now GlaxoSmithKline ) in 1971 ; the more water-soluble and less toxic hydrochloride is used .
Clinical information
application
Labetalol is used in arterial hypertension for long-term medication in tablet form as well as intravenously and in emergency medicine exclusively iv. It acts as an antagonist on both alpha-adrenoceptors and beta-adrenoceptors (β1 and β2).
Side effects
Side effects include arterial hypotension , lethargy , insomnia, erectile dysfunction, nausea, difficulty urinating, visual disturbances, nasal congestion and hypersensitivity reactions.
Contraindications
A relative contraindication is given in patients with asthma , congestive heart failure, any blockage of the cardiac conduction system (including SA block and AV block ), bradycardia, and cardiogenic shock .
Stereochemistry
Labetalol contains two stereocenters and consists of four stereoisomers. This is a mixture of ( R , R ) -, ( S , R ) -, ( R , S ) - and the ( S , S ) form:
| Stereoisomers of Labetalol | |
|---|---|
 CAS number: 75659-07-3 |
 CAS number: 83167-24-2 |
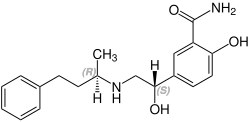 CAS number: 83167-32-2 |
 CAS number: 83167-31-1 |
Trade names
Monopreparations : Trandate (A, CH)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d e Entry on Labetalol. In: Römpp Online . Georg Thieme Verlag, accessed on August 5, 2013.
- ^ Hermann J. Roth and Helmut Fenner, Pharmaceuticals , Deutscher Apotheker Verlag, Stuttgart 2000.
- ↑ a b Red List 1988 , Federal Association of the Pharmaceutical Industry eV, Editio Cantor, Aulendorf, 1988.