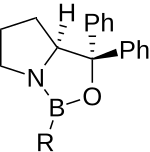
CBS reduction
The CBS reduction or Corey-Bakshi-Shibata reduction is an enantioselective reduction of ketones to the homochiral secondary alcohols developed by the chemists Corey , Bakshi and Shibata . The CBS catalyst itself is a chiral auxiliary which is chemically derived from the amino acid proline .
The CBS catalyst is used in this reaction as a chiral catalyst in the reduction of prochiral ketones to secondary chiral alcohols. By using ( S ) -CBS or ( R ) -CBS, both enantiomers of the alcohol are possible through this reduction.
The reducing agent which is used in stoichiometric amount is borane (BH 3 ). Borane itself only reacts slowly and, above all, unselectively with ketones . The CBS catalyst serves on the one hand for activation and on the other hand to provide a chiral environment. It is therefore a so-called ligand-accelerated reaction. The hydride source borane is activated by binding to the nitrogen atom of the catalyst and by complexation to the carbonyl oxygen atom . First, a Lewis acid-base complex is formed from the CBS catalyst and the carbonyl group. The electrophilicity of the carbonyl group is increased enough that a reduction with borane is possible. Now the hydride atoms can attack nucleophilically on the carbonyl carbon atom and enable the reduction. The CBS catalyst ensures a clear fixation of the transition state and the hydride ion will preferentially attack from the sterically preferred conformation.
Individual evidence
- ↑ EJ Corey, Saizo Shibata, Raman K. Bakshi: An efficient and catalytically enantioselective route to (S) - (-) - phenyloxirane . In: The Journal of Organic Chemistry . tape 53 , no. 12 , 1988, pp. 2861-2863 , doi : 10.1021 / jo00247a044 .
- ↑ Lyndon C. Xavier, Julie J. Mohan, David J. Mathre, Andrew S. Thompson, James D. Carroll, Edward G. Corley, and Richard Desmond: (S) -Tetrahydro-1-methyl-3,3-diphenyl -1H, 3H-Pyrrolo- [1,2-c] [1,3,2] Oxazaborole-Borane Complex In: Organic Syntheses . 74, 1997, p. 50, doi : 10.15227 / orgsyn.074.0050 ; Coll. Vol. 9, 1998, p. 676 ( PDF ).
- ↑ Scott E. Denmark, Lawrence R. Marcin, Mark E. Schnute, and Atli Thorarensen: (R) - (-) - 2,2-Diphenylcyclopentanol In: Organic Syntheses . 74, 1997, p. 33, doi : 10.15227 / orgsyn.074.0033 ; Coll. Vol. 9, 1998, p. 362 ( PDF ).
- ^ Reaction mechanism Corey-Bakshi-Shibata Reduction. In: Organic Chemistry Portal. Accessed December 2, 2018 .
- ↑ Examples of the CBS reduction: Gerhard Bringmann, Matthias Breuning, Petra Henschel, and Jürgen Hinrichs: Asymmetric Synthesis of (M) -2-Hydroxymethyl-1- (2-Hydroxy-4,6-Dimethylphenyl) NNaphthalene via a configurationally unstable Biaryl Lactone In: Organic Syntheses . 79, 2002, p. 72, doi : 10.15227 / orgsyn.079.0072 ; Coll. Vol. 10, 2004, p. 448 ( PDF ).