Auxiliary
An auxiliary (lat. Auxiliari = help) referred to in the Chemistry a covalently to a molecule attached group that enables a particular reaction or its stereochemical influenced history. After a successful reaction, the auxiliary can be split off again in a further step.
Chiral auxiliaries
With the help of a chiral auxiliary, the course of a chemical reaction that is not stereoselective in and of itself can be controlled in such a way that, after the auxiliary has been split off, one enantiomer is still preferably obtained. The best-known example of a chiral auxiliary is the Evans auxiliary, which is used in the Evans synthesis of enantiomerically pure α-alkylated carboxylic acid derivatives . Chiral auxiliaries are the most important auxiliaries used in organic synthesis.
The functionality of an auxiliary is based on the fact that stereochemical information is brought into the molecule by attaching a chiral group . As a result, possible attacks on this molecule are always diastereoselective, so the two products of the reaction are diastereomers . A diastereomer is preferably formed by steric interaction (for example shielding one side of the molecule by a large substituent on the auxiliary). After the auxiliary has been split off, the diastereomers are converted into enantiomers due to the loss of the stereogenic center on the auxiliary.
The advantage of using chiral auxiliaries is the relatively easy access from the chiral pool . The auxiliaries known and commonly used today have been tried and tested and mostly give very good stereoselectivities . The disadvantage is that, in contrast to stereoselective catalysis , the stereochemical information has to be used stoichiometrically and the auxiliary often cannot be recovered after the cleavage. The atom economy when using an auxiliary in a reaction sequence is therefore usually moderate.
Evans auxiliaries
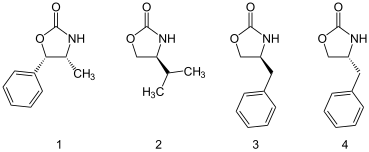
structure
Evans auxiliaries are oxazolidinones . The auxiliary 2 is derived from valine . The benzyl-substituted auxiliaries 3 and 4, which can be obtained from phenylalanine , are most frequently used today . They are named after their discoverer, David Evans .
synthesis
Evans auxiliaries are derived from amino acids . These are first reduced to the amino alcohol and then condensed to the oxazolidinone .
use
Without the use of an auxiliary, the attack of an electrophile (in the example methyl iodide ) on the enolate ( generated from the methyl ester by deprotonation with LDA ) takes place unselectively from both sides of the double bond, whereby both enantiomers are formed in the same ratio (ee = 0%) become.
However, if the Evans auxiliary (a chiral oxazolidinone that is bound to the molecule via an amide bond) is attached instead of the methyl ester , the attack takes place from the sterically less hindered side of the molecule. Due to the stereocenter in the auxiliary, the reaction is now diastereoselective. The two possible products of the alkylation (attack from above and below) produce two diastereomers , which can also be separated if necessary. Only after the auxiliary has been split off (here with LiOH / H 2 O 2 ) is the desired product obtained in enantiomerically pure form.
Enders reagent
( S ) -1-Amino-2- (methoxymethyl) pyrrolidine and ( R ) -1-Amino-2- (methoxymethyl) pyrrolidine (SAMP and RAMP) are referred to as Enders reagents . They are auxiliaries derived from proline that can be used for the stereoselective alkylation of aldehydes (so-called RAMP or SAMP process).
Other auxiliaries
8-Phenylmenthyl auxiliary: For auxiliary-induced diastereoselectivity in conjugate additions of cuprates .
2,5-Dimethylpyrrolidine is used, among other things, as an auxiliary for intermolecular radical additions to acrylic acid derivatives.
literature
- DA Evans, Studies in Asymmetric Synthesis - The Development of Practical Chiral Enolate Synthons In: Aldrichimica Acta . Vol. 15, 1982, p. 23.
- R. Brückner, Reaction Mechanisms . Elsevier, Heidelberg 2004, ISBN 978-3-8274-1579-0 .
- Key Chiral Auxiliary Applications . (Second Edition) (ed .: G. Roos), Academic Press, Boston, 2014, ISBN 978-0-12-417034-6 .