Enders reagent
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Enders reagent | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 6 H 14 N 2 O | |||||||||
| Brief description |
light yellow liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 130.21 g mol −1 | |||||||||
| density |
0.97 g cm −3 (25 ° C) |
|||||||||
| boiling point |
186-187 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
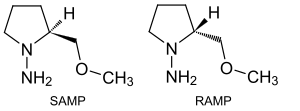
The two stereoisomeric chemical compounds ( S ) - (-) - and ( R ) - (+) - 1-amino-2-methoxymethylpyrrolidine , which are often abbreviated to SAMP or RAMP, are called Enders reagent . The two nitrogen heterocycles are named after Dieter Enders . They are used as chiral auxiliaries , i.e. H. as auxiliaries which, through their own stereochemical information in a chemical synthesis, control the reaction to a desired end product with a fixed stereochemistry and then (can) be split off again.
presentation
SAMP is produced in a four-step synthesis from L - proline [synonym: ( S ) -proline]. The enantiomer of SAMP - i.e. ( R ) - (+) - 1-amino-2-methoxymethylpyrrolidine (RAMP) - can be prepared from D- proline [synonym: ( R ) -proline] or ( R ) -glutamic acid in a six-step synthesis become.
Analytics
A gas chromatographic method for determining the enantiomeric purity of SAMP and RAMP has been described in the literature.
use
SAMP and RAMP are used as chiral auxiliaries in enantioselective synthesis. Using the SAMP / RAMP hydrazone method, ketones and aldehydes which are chirally alkylated in the α-position can be synthesized with good enantiomeric and diastereomeric excesses . The reaction is based on the formation of the hydrazone from the chiral auxiliary and the starting material, a ketone or aldehyde . The oxygen of the methoxy group has a chelating effect on the lithium ion of enolate-forming bases such as lithium diisopropylamide . This induces the stereo information, the position of the methoxy group, on the product. The intermediate products are hydrazones, which are converted into the end products by means of ozonolysis . The auxiliary is obtained as a nitro derivative and can be reduced, i.e. recycled, to hydrazine (SAMP or RAMP) with zinc and acetic acid . Many natural substances , especially pheromones , e.g. B. ( S ) - (+) - 4-methyl-3-heptanone, an alarm pheromone of leaf cutter ants , could be successfully synthesized.
literature
- Dieter Enders, Karl-Erich Jaeger: Asymmetric Synthesis with Chemical and Biological Methods . Wiley-VCH publishing house, ISBN 3-527-31473-3 .
Web links
- Dieter Enders, Peter Fey, and Helmut Kipphardt: (S) - (-) - 1-Amino-2-Methoxymethylpyrrolidine (SAMP) AND (R) - (+) - 1-Amino-2-methoxymethylpyrrolidine (RAMP), Versatile chiral Auxiliaries In: Organic Syntheses . 65, 1987, p. 173, doi : 10.15227 / orgsyn.065.0173 ; Coll. Vol. 8, 1993, p. 26 ( PDF ).
Individual evidence
- ↑ a b c Data sheet (S) - (-) - 1-Amino-2- (methoxymethyl) pyrrolidine from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b RAMP data sheet (PDF) from Merck , accessed on April 9, 2010.
- ↑ Data sheet (R) - (+) - 1-Amino-2- (methoxymethyl) pyrrolidine from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b A. Job, CF Janeck, W. Bettray, R. Peters, D. Enders: The SAMP / RAMP-Hydrazone Methodology in Asymmetric Synthesis , in: Tetrahedron , 2002 , 58 , pp. 2253–2329. doi : 10.1016 / S0040-4020 (02) 00080-7
- ↑ Kurt Günther, Jürgen Martens and Maren Messerschmidt: Gas Chromatographic Separation of Enantiomers: Determination of the Optical Purity of the Chiral Auxiliaries (R) - and (S) -1-Amino-2-methoxymethylpyrrolidine , in: Journal of Chromatography A , 1984 , 288 , pp. 203-205, doi : 10.1016 / S0021-9673 (01) 93696-9 .
- ↑ Dieter Enders, Helmut Kipphardt, and Peter Fey: Asymmetric Syntheses using the SAMP- / RAMP-Hydrazone Method: (S) - (+) - 4-Methyl-3-Heptanone In: Organic Syntheses . 65, 1987, p. 183, doi : 10.15227 / orgsyn.065.0183 ; Coll. Vol. 8, 1993, p. 403 ( PDF ).