Natural substance
Natural Products ( English natural product in) denotes Chemistry a compound derived from organisms is formed to meet biological functions; a modern synonym for natural substance in the sense of chemistry is biomolecule . Only pure substances or defined mixtures of substances are understood as such. The legislator defines organic substances by means of the Biological Agents Ordinance .
In common parlance, natural substance is a broader term: all substances that humans have not produced artificially. This article only relates to the specific term defined above in the sense of chemistry. After that, they do not count among the natural substances
- Complex, non-pure substances formed by living beings, natural products such as feathers , wood or cotton that are made up of natural substances but are non-uniform mixtures of substances
- all naturally occurring inorganic substances such as minerals or rocks
Although a natural substance is a defined compound, it occurs in organisms in many mutually convertible modifications. The biological function is controlled or controlled by the modifications. After extraction and purification, the stable basic structure is usually obtained, which can be assigned to the classes of natural substances.
Natural substances are built up in all living organisms or converted into one another. Their synthesis is associated with an energy expenditure for the organism. Their tasks are varied depending on the substance class and range from simple metabolism or energy generation to cell components and building materials of the organism to complex control tasks . With regard to their functions, a distinction can be made between primary and secondary natural substances. The primary natural substances include all compounds that are required for the organism to support life and growth. These include above all the fats and the biopolymers of carbohydrates and proteins . The secondary natural substances are formed for reasons that are often as yet unknown and are divided into the large substance classes of terpenes , aromatics and alkaloids .
The chemistry of natural substances is an interdisciplinary science that can use methods of organic and analytical chemistry to answer questions of biology , biochemistry , physiology and pharmacy . Natural product chemistry is of great importance in pharmacology , in the development of new active ingredients and generally in method development in these disciplines.
history
The original term natural product was determined by the historical definition of organic chemistry ; it comprised the entirety of the substances that are used in the structure of animals and plants . Even Jons Jakob Berzelius took 1827 due to the state of knowledge and the complex chemical structure of natural substances that there is a generation for their life force ( vis vitalis had to be). The distinction between self-organized and externally organized substances was revised by Friedrich Wöhler (1828), who had demonstrated with urea synthesis that the compound, defined as inorganic , ammonium cyanate, can be converted into the compound, defined as organic , urea.
In today's definition, organic chemistry includes practically all carbon compounds. In the course of time, natural product chemistry developed into its sub-area and deals with the isolation, structure elucidation , synthesis or biosynthesis and the properties of compounds that occur in organisms (such as animals, plants and microorganisms).
Initially, natural product chemistry only dealt with ingredients of plant origin, as it was very much influenced by pharmacognosy (drug science). Compounds (initially mostly alkaloids ) were isolated from plant extracts and attempts were made to clarify their structure.
In the middle of the 19th century, Justus Liebig expanded the concept of natural substances to include compounds of animal origin. Emil Fischer became a pioneer in the structure elucidation and synthesis of carbohydrates and proteins towards the end of the 19th century .
By the end of the 1930s, the most important classes of natural substances were found, examined and their structure clarified. Important milestones are here:
- Terpenes by Otto Wallach from essential oils
- Steroids by Adolf Windaus and Heinrich Otto Wieland
- Carotenoids by Paul Karrer
- Porphine dyes by Richard Willstätter and Hans Fischer
- Vitamins by Paul Karrer , Adolf Windaus , Robert R. Williams , Richard Kuhn and Albert von Szent-Györgyi Nagyrápolt, among others
- Hormones by Adolf Butenandt and Edward Calvin Kendall
With the discovery of penicillin in 1940 by Alexander Fleming , Ernst Boris Chain and Howard Walter Florey , microorganisms were also recognized as a profitable source of natural substances.
After the end of the Second World War, natural product chemistry was promoted by the development of new and very powerful analytical and physical methods. Both the mass spectrometry , X-ray crystallography and later the NMR spectroscopy allowed hitherto unimagined possibilities of structure determination without derivatization of the natural product and small amounts of analyte . The only now slowly established methods of chromatography and electrophoresis made it possible to separate the substance mixtures at a previously unattainable speed and purity.
Definition in chemicals law
The term natural substance is legally defined as "naturally occurring substance as such, unprocessed or only processed manually, mechanically or by gravitational force, by dissolution in water, by flotation, by extraction with water, by steam distillation or by heating to remove water or by any means taken from the air " .
According to Annex V paragraphs 7 and 8 of the REACH regulation (EG 1907/2006), natural substances are exempt from registration if they have not been chemically modified. This does not apply if they are classified as dangerous according to the criteria of the CLP Regulation (EC No. 1272/2008), meet the criteria for PBT or vPvB substances or are just as worrying.
Definition from the Biological Agents Ordinance
According to the BioStoffV, biological agents are essentially microorganisms, protein structures that can cause diseases and cell cultures.
"In addition to the European Directive 2000/54 / EC (protection of workers from the risks posed by biological agents at work), the Biological Agents Ordinance also sets the European Directive 2010/32 / EU (avoidance of injuries from sharp / pointed instruments in the hospital and health sector) into German labor protection law.
The Biological Agents Ordinance regulates occupational activities with biological agents, ie in the broadest sense with microorganisms / pathogens. It contains regulations for the protection of employees during these activities, i. H. to protect against infections and against sensitizing, toxic or other health-damaging effects. The Biological Agents Ordinance divides biological agents into four risk groups. The risk assessment and the definition of the necessary measures are carried out on this basis. "
Importance of natural product chemistry
pharmacology
Since Alexander Fleming's discovery of penicillin, natural substances have become an important source of lead structures for active pharmaceutical ingredients. Natural substances have pharmacological effects as antibiotics , immunosuppressants , enzyme inhibitors , receptor antagonists and agonists , toxins , antitumoral and antiviral agents.
A whole range of active ingredients are derived from natural substances today. In addition to β-lactam antibiotics, this also includes chemotherapy drugs such as paclitaxel from the Pacific yew tree ( Taxus brevifolia ) or epothilone from the myxobacterium Sorangium cellulosum .
Nucleosides have been modified so that they can be used as antivirals , such as the HIV drug zidovudine .
Centuries of experience in folk medicine has drawn attention to many plants and thus to their ingredients as lead structures. For example, ginseng ( Panax ginseng ), ginkgo ( Ginkgo biloba ) or neem tree ( Azadirachta indica ) have long been the subject of intensive research.
The lead structures obtained in this way serve as the basis for the pharmacological optimization of the active ingredient. Here are structure-activity relationships established (QSAR), and tries to optimize the physical properties such as solubility in aqueous media. Techniques such as parallel synthesis or combinatorial chemistry are often used for this purpose .
Natural substances are referred to pharmacologically as privileged structures because they are formed under physiological conditions and show advantageous pharmacokinetic properties. The fact that around half of the best-selling active ingredients are natural substances or their derivatives shows the special importance of natural substances for pharmacology.
biology
The elucidation of physiological relationships is important for biology . In the field of natural substances, this includes biosynthesis and the biological function in organisms, be it as an enzyme , messenger substance or as an energy supplier or store. The messenger substances include a. Hormones , neurotransmitters and pheromones . Energy suppliers and stores are usually fats, proteins and carbohydrates. The biosynthesis of natural substances is as diverse as their variance in structure.
The elucidation of a biosynthetic pathway often requires great effort. Different techniques are used:
- Isotope technology - Potential precursor molecules are marked with a rare, possibly radioactive, but not too rapidly decaying isotope , such as the 14 C isotope (half-life 5736 years). After it has been introduced into the metabolism (e.g. through feeding or injection), the fate of the isotope in the target molecule is observed. From this one can deduce the path of biosynthesis.
- Enzymatic techniques - here the biosynthetic pathway must be approximately known. One works on isolated enzymes or with cell cultures in order to study biosynthetic pathways under laboratory conditions and no longer in vivo .
- Genetic engineering methods - for example, here the biosynthesis by bacteria is interrupted with the help of gene mutations; an intermediate of a biosynthetic sequence accumulates in the process.
Organic chemistry
For organic chemistry, natural product chemistry represents a challenge in many respects. On the one hand, in the area of structure elucidation, i.e. analysis: it is not uncommon for several working groups to work on structure elucidation for years. Complex structures such as B. Azadirachtin required several attempts before the correct structure was proven. Even the total synthesis of a natural product does not always prove the correct structure - as has been shown in patchulia alcohol and the synthesis by Büchi.
In general, the synthesis of a complex natural substance is also a challenge for organic chemistry. In order to gain importance as a pharmaceutical , a total or partial synthesis has to be developed for a natural product . This is even more important in the context of structure optimization, because thousands of compounds have to be synthesized on the basis of a natural substance in order to establish a structure-activity relationship and to be able to optimize the pharmacological properties. Natural products are usually complex compounds with centers of chirality , which have to be built up in the desired stereochemical configuration. The reagents used must be compatible with the functional groups in the molecule, or an appropriate protective group strategy must also be selected.
The total syntheses of vitamin B 12 by Robert B. Woodward and Albert Eschenmoser in 1973, the synthesis of palytoxin by Yoshito Kishi in 1994 or the race for the first total synthesis of taxol show how long and labor-intensive a natural product synthesis can be between Robert A. Holton , Kyriacos C. Nicolaou and Samuel J. Danishefsky from the same year. The synthesis of vitamin B 12 took about 20 years of development work. For this purpose, completely new reaction steps had to be developed and, for vitamin B 12 , the Woodward-Hoffmann rules even created new theoretical foundations. Roald Hoffmann was awarded the Nobel Prize for their development . Robert B. Woodward had already received an award in 1965 for his work in the field of natural product chemistry.
Another importance of natural substances in organic chemistry is their use as a source of synthetic building blocks. Many natural substances, such as B. sugars or amino acids are chiral compounds and can thus be used as precursor molecules for chiral syntheses or as reagents. However, natural products can also simply be a source for complex starting compounds and even for industrial syntheses. For example, shikimic acid is the starting material for the large-scale synthesis of the influenza active ingredient oseltamivir (Tamiflu) from Roche .
Classification according to biological function
When classifying natural substances according to their biological function, a distinction is made between primary natural substances and secondary natural substances . The distinction goes back to the Nobel Prize winner Albrecht Kossel .
This classification is now rather arbitrary and historical, but is still used in the literature. This classification is obsolete in terms of both chemical structure and biological function, as a natural substance can have both a life-sustaining function in the sense of Kossel, but can also have classic functions of secondary natural substances (transmitter molecules, pheromones, repellants, etc.).
Primary natural substances
According to A. Kossel's definition, primary natural substances include all compounds that are necessary in the organism for life support and growth. However, this is not a strictly delimited class and the transitions between the primary and secondary metabolic pathways are fluid.
Primary natural substances are found during the development ( growth ) of living beings, but also during the breakdown into smaller molecules, which can be associated with a gain in energy for the organism. This energy can in turn be used to build other primary or secondary biomolecules. The build-up and breakdown of natural substances are the basis for the energy and mass metabolism in all organisms.
Secondary natural substances
Secondary natural substances are formed for many reasons, but they are not essential for the life of the organism. As secondary plant substances in particular, they represent a very large variety of chemical structures and are formed in what is known as secondary metabolism. This follows on from the primary metabolism and therefore cannot take place independently of it. However, the secondary metabolism is not involved in the energy metabolism and is neither part of the anabolic (building) nor the catabolic (breaking down) metabolism. Secondary natural substances are only formed in special cell types . The transitions from primary metabolic products to secondary metabolic products are fluid. The biological function of secondary natural substances is very diverse and often not known.
Classification according to chemical structure
When classifying natural substances according to their chemical structure ( substance class ), a distinction is made between biomolecules and biomolecule groups according to their functional groups , i.e. H. their structural design. In the case of natural substances one can find both small, simple molecules such as B. steroids , aromatic compounds or fatty acids , but also very complex biopolymers such as proteins , DNA and carbohydrates . This classification is not to be regarded as absolute either, since complex substances, e.g. B. a highly glycosylated protein ( glycoprotein ), cannot be assigned to a pure chemical class of substances.
Amino acids, peptides and proteins
The proteinogenic amino acids are exclusively α-amino acids. However, β-amino acids such as β-alanine, β-aminobutyric acid or γ-aminobutyric acid also occur naturally. All naturally occurring α-amino acids (with the exception of glycine ) are chiral . It is virtually exclusively to L -amino acids.
The complete synthesis of all 20 biogenic canonical amino acids can only be found in microorganisms and plants. Animals - including humans - have to take in certain amino acids - in humans these are valine , leucine , isoleucine , methionine , threonine , lysine , phenylalanine and tryptophan , and in childhood also tyrosine - as essential amino acids with their food. For fish and insects, arginine and histidine are also essential.
The proteinogenic amino acids form the basis for all variants of amino acids. Other amino acids such as B. Ornithine or homoserine occur in proteins and as metabolic products. Other non-proteinogenic amino acids are formed by hydroxylation of proteinogenic amino acids. This includes B. 4-hydroxyproline . Products from N -methylation or iodination can also be found. Some halogenated amino acids have been found in mollusks . In total, over 400 amino acids have been identified so far that are not incorporated into proteins. Many of these are formed by the hydroxylation or methylation of homologous proteinogenic amino acids. They occur in peptide antibiotics or as toxins (such as in the death cap mushroom ). Rare amino acids such as canavanine ( Fabaceae ), mimosine ( mimosa species) and 2-methylene-cyclopropylglycine ( Sapindaceae ) act as antagonists of the structurally related amino acids arginine , phenylalanine and tyrosine or leucine and are therefore toxic.
Peptides and proteins
Both peptides and proteins are chains of amino acids that are linked to one another via an amide bond . A distinction is made here between oligopeptides , peptides and proteins depending on the number of amino acids and molar mass .
| Surname | Number of amino acids | molar mass |
|---|---|---|
| Oligopeptide | 2-10 amino acids | |
| peptide | > 10 to about 80-90 amino acids | |
| protein | from about 80-90 amino acids | from 10,000 Da or 10 kDa |

The division between peptide and protein is based on the fact that proteins can not pass through dialysis membranes due to their high molar mass . Since the molecular masses of proteins are quite large, the unit of measurement commonly used here is kilodalton (unit symbol kDa), which corresponds to the normal unit of mass for atoms and molecules, but has the prefix “kilo” (and thus a factor of 10 3 ) added .
Due to the vectorial linkage of an acylic peptide or protein, a distinction is made between the two ends, the N terminus (the end with a free or modified amino group) and the C terminus (the end with a free carboxylate group).
Peptides that are built up only from amino acids are called homeomeric peptides. Peptides that also contain pseudo amino acids are called heteromeric peptides. The pseudoamino acids are z. B. calculated hydroxycarboxylic acids , which interrupt the alternating amide structure of a peptide by an ester bond .
Cyclic peptides are also called peptolides. Depending on whether the amino acids in a peptide are only linked to one another via amide bonds or whether there are other bonds such as disulfide bridges , one speaks in the first case of homodetic peptides and otherwise of herodetic peptides. Many peptides have a strictly linear structure - but there are also branched peptides that are formed by reactions on the side chains. The ribonucleases are an example of branched peptides.
Due to their modular structure, proteins and peptides are very variable in their physical properties and therefore have very special and very different functions in organisms . The important tasks of proteins include acting as enzymes , i.e. they catalyze biochemical reactions, are toxins to defend against hostile organisms, are an important part of the immune system , they form body structures such as muscles and are transmitter molecules.
carbohydrates
A distinction is made within the carbohydrates between monosaccharides , oligosaccharides and polysaccharides according to the following scheme:
| Surname | Number of monosaccharide units |
Examples |
|---|---|---|
| Monosaccharides | 1 | Glucose , fructose |
| Oligosaccharides | 2-9 | Sucrose , maltose , raffinose |
| Polysaccharides | > 10 | Starch , cellulose |
In the case of polysaccharides, a distinction is made between homopolysaccharides such as starch, which is made up of an alternating sugar unit, and heteropolysaccharides, which contain various sugars.
Carbohydrates have a variety of functions in the organism. They are an energy store, which can be mobilized very quickly, form the exoskeleton of the arthropods in the form of chitins , as cellulose they are an important component of the cell walls of plants and with their starch they are an energy store for plants and thus also an important energy supplier for animal and human consumption.
Monosaccharides
The most common monosaccharides are the aldohexoses and pentoses and their 2-keto variants. Of these, glucose has a central and important role in the metabolism of carbohydrates and thus also in the energy balance of organisms. The breakdown of monosaccharides for energy production in the form of adenosine triphosphate (ATP) is called glycolysis . It takes place in practically all organisms in the same form.
All monosaccharides are chiral compounds and practically all naturally occurring monosaccharides come from the D series. If a hydroxyl group has been removed in the metabolism , one speaks of deoxy sugars. The deoxy sugars are mostly deoxyl al doses, which are usually found in glycosidic bonds . One example of this is deoxyribose as a component of deoxyribonucleic acid (DNA). Branched deoxy sugars are also known as methylose and are important as a blood group substance or in cardiac glycosides . They are formed in the organism through carbon transfer or rearrangement reactions .
In addition to the monosaccharides, which have no other heteroatom apart from oxygen, the amino sugars are important. Glycosidically bound, they are part of antibiotics, part of the murein in the cell walls of bacteria and the building blocks of chitin armor.
Cladinose , an example of methylosis
Monosaccharides occur free in nature, but also in bound form as carbohydrates, as the sugar component of a glycoside and as esters of inorganic acids such as phosphoric acid or monosulfuric acid.
They are built up in plants from carbon dioxide and water in the Calvin cycle of the photosynthesis process . In the event of an insufficient intake of carbohydrates, animals and humans have to resort to amino acids in order to synthesize monosaccharides from them. However, this process is associated with an increased expenditure of energy. The various monosaccharides can be converted into each other by all organisms, so that in contrast to the fatty acids and amino acids, no essential sugars are known.
Cyclitols
The cyclitols are closely related to the monosaccharides. This is understood to mean cycloalkanes with at least three hydroxyl groups. The most common representatives here are the hexahydroxycyclohexanes, which are also called inosites . They come in free form or phosphorylated . More recently, their role as was a second messenger ( second messenger ) detected. By substituting one or more hydroxyl groups with an amino group, aminodeoxyinosites are obtained.
Di- and oligosaccharides
Oligosaccharides are made up of two or more sugar units and are accordingly referred to as di-, tri-, tetrasaccharides, etc. By far the most common disaccharide is sucrose (cane or beet sugar), which consists of a glucose and a fructose unit.
Sucrose is found in many plants. It is obtained industrially from cultivated forms of sugar cane ( Saccharum officinarum , 14–20% content) and sugar beet ( Beta vulgaris , 16–20% content).
Another very important disaccharide is lactose , which is practically the only source of carbohydrates in the diet of newborns of mammals (Mammalia). Lactose consists of 1,4-linked galactose with glucose . Other important representatives of the disaccharides are trehalose (insects, fungi, yeast, algae, bacteria and moss), gentiobiosis (e.g. as the sugar residue of amygdalin - the glycoside of bitter almonds ( Prunus amygdalus amara )) and primaverine (from primroses ( Primula )) .
Polysaccharides
Polysaccharides are ubiquitous natural substances. An important polysaccharide is starch , a vegetable reserve substance that is of great importance for human and animal nutrition. Polysaccharides serve as reserve material or form the structure of cells or organisms. They also form the basis for the cell walls that surround the cells of bacteria and plants. As a component of the cell wall in plants, cellulose is of outstanding importance, which also plays an important role in the nutrition of ruminants . Other important polysaccharides that serve as cell wall building blocks in plants are pectins and hemicelluloses . Containing polysaccharides which aminosugars are used in animals and fungi in the form of chitin before, which is a homopolysaccharide of N -acetyl glucosamine is.
A number of polysaccharides have native or chemically modified significance as additives in the food, pharmaceutical, textile and cosmetics industries. They are obtained either from plant material or biotechnologically . These include xanthan , dextran , levan and pullulan .
Glycosides
Glycosides are conjugates of mono- or oligosaccharides with alcohols, thiols , aldehydes or amides , which are linked directly via the anomeric carbon atom or via a heteroatom. C- glycosides are also known in which there is a pure carbon-carbon bond because the anomeric hydroxyl group was removed before the linkage. Depending on the type of bond, one speaks of O , S , N or C glycosides.
Strictly speaking, oligo- and polysaccharides are also glycosides, but the term glycoside is usually only used for conjugates with non-carbohydrate residues. This residue is known as the aglycon. A variety of compounds have been found for the aglycon. Only a few important representatives are listed here as examples.
| Functional group of the aglycone bonded to the sugar | Surname | structure | Occurrence |
|---|---|---|---|
| phenol | Arbutin | 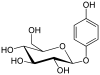 |
Arbutin is a simple glycoside and occurs in a wide variety of fruits. The aglycon is a hydroquinone and the sugar residue is a β-glucose. |
| alcohol | Oleandrin | 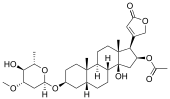 |
Oleandrin is an ingredient of the oleander ( Nerium oleander ) and has a steroid as an aglycon |
| alcohol | Digitoxin |  |
Digitoxin is an ingredient of the red foxglove ( Digitalis purpurea ) and has a steroid as an aglycon |
| Thiocarboxamide | Sinigrin | 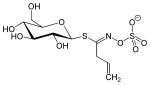 |
Sinigrin is an ingredient of black mustard ( Brassica nigra ) and horseradish ( Armoracia rusticana ) and has an allyl thiocarboxamide as an aglycon |
| Aldehyde (cyanohydrin) | Amygdalin |  |
Amygdalin is the cyanogenic glycoside of the bitter almond ( Prunus amygdalus amara ) and carries the cyanohydrin of benzaldehyde as an aglycon |
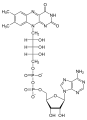
| Nitrogen heterocycle | Adenosine |  |
Adenosine is a building block of DNA and has a purine base as an aglycon |
| Anthraquinone | Barbaloin |  |
Barbaloin occurs among other things in different types of aloe ( aloe ) and carries an anthraquinone derivative as an aglycon |
Glycosides are mainly used as cardiac glycosides (Digitoxin) or as antibiotics ( Erythromycin ) and are obtained from natural sources. Biologically, they are of indispensable importance as building blocks of DNA and RNA.
The formation of glycosides in organisms often serves to bring a rather apolar aglycon into a water-soluble form.
Peptidoglycans
Peptidoglycans, also called murein, are conjugates of polysaccharides with peptides. They give the cell walls of bacteria their strength. They consist of a disaccharide ( N -acetylglucosamine β- (1,4) -linked with N -acetylmuramic acid ), which forms a polysaccharide and is cross-linked via short peptide chains. The cross-linking is formed by a transpeptidase , which can be inhibited by antibiotics and thus prevent the build-up of stable cell membranes.
Lipids
Lipids is a collective term for non-polar compounds that can be extracted from organic material with non-polar organic solvents such as ether , petroleum ether or chloroform . This term is purely historical, as it extracts compounds that have no structural similarity to one another (such as terpenes or steroids ), while others are structurally similar to fats - such as glycolipids .
Today lipids are compounds that are derived from fats , i.e. esters of fatty acids with monohydric or polyhydric alcohols.
Fatty acids

The most common natural fatty acids are long-chain carboxylic acids with an even number of carbon atoms. A distinction is made between saturated and unsaturated fatty acids, i.e. without or with (one or more) double bonds in the alkyl chain. The double bonds of natural fatty acids are always Z -configured ( cis -configuration).
Some of the unsaturated fatty acids are essential for humans, as they cannot be synthesized by the body and must therefore be ingested with food. They are partly as vitamin F , respectively.
In nature, fatty acids are rarely free, but are usually linked to alcohols via an ester bond. The most common alcohol component is glycerine (glycerolipids). Esters with amino alcohols (sphingolipids), monosaccharides (glycolipids), diols (diol lipids) and myo-inositol are also known .
Branched, longer-chain aliphatic carboxylic acids, due to their completely different biosynthesis, are not counted among the fatty acids but among the terpenes .
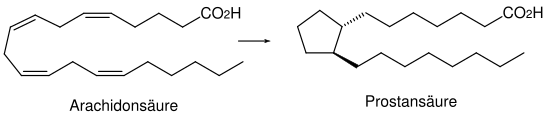
Eicosanoids
Unsaturated fatty acids form the starting compounds for a large number of regulating substances. The basis for this is arachidonic acid , which is an unsaturated fatty acid and contains twenty carbon atoms. Hence the name eicosanoids is derived . The arachidonic acid is formed in the organism from the essential linoleic acid through chain extension and dehydration.
The framework of the prostaglandins , which are the most important eicosanoids, is derived from prostanoic acid . They always consist of a five-membered ring with two adjacent side chains. Both the side chains and the five-membered ring can carry different functional groups.
The analogous compounds with a six-membered ring are called thromboxanes .
Fats

Fats are the esters of fatty acids with glycerine. These are mostly triglycerides, because mono- and diglycerides only play a role as metabolic intermediates and are rarely free. Since glycerine is a trihydric alcohol, in addition to esters of three molecules of the same fatty acid, mixed esters are also found. If this gives the glycerol residue a center of asymmetry, fats are chiral compounds and optically active through biosynthesis via L- glycerol-3-phosphate (G3P).
In all animals, fats are used as energy stores in specialized tissues; adipose tissue can contain up to 80% fat. The seeds of various plants also store fats.
It is usually not possible to separate different fats, as they are multiple mixtures of substances with very similar chemical and physical properties.
Waxes
Waxes are non-polar esters of fatty acids and cyclic or long-chain aliphatic alcohols. Naturally occurring waxes are usually difficult to separate mixtures of substances, which are more of technical importance. Waxes usually serve as structure builders, such as in honeycombs, and can usually no longer be fed into the metabolism. Exceptions are certain marine animals that produce wax as reserve materials, such as B. Whale Rat ( Cetaceum ). In plants, waxes form the cuticle as protection against evaporation. The alkyl radical of the alcohol group can be branched or unbranched. In mammals, the alcohol group mostly consists of cholesterol .
Complex lipids
As complex lipids or membrane-forming lipids , such lipids are called, which are involved in the synthesis of cell membranes. In addition to the non-polar fatty acid residues, these lipids have polar groups. These polar groups give them the ability to self-organize in aqueous media. This can be demonstrated under laboratory conditions by the formation of liposomes . A similar structure can be found in cell membranes. In aqueous media the polar groups organize themselves in the direction of the polar water and the non-polar groups form a lipid bilayer - with water outside and inside the lipid bilayer.

Phospholipids , sphingolipids and glycolipids are lipids that form cell membranes. They differ in their polar residues. In the case of phospholipids, it is a phosphoric acid ester of diglyceride. There are still polar residues on the phosphate residue such as choline ( lecithin ) or ethanolamine ( cephalin ). In contrast to the phospholipids, sphingolipids are derived from sphingosine . The fatty acid is bound to sphingosine via an amide bond, which in turn is connected to a polar group such as serine , ethanolamine or choline via a phosphate residue through ester bonds . Glycolipids, on the other hand, are various fatty acid derivatives with a sugar group as a polar residue. These can either be of the glyceride type, of the sphingolipid type or simple fatty acid esters of the monosaccharides.
Isoprenoid compounds
Isoprenoid compounds are derived from isoprene and are formally oligomers or polymers of isoprene. This principle was recognized by the Nobel Prize winner Leopold Ružička . From this the group of natural substances called terpenes and steroids is formed. The latter are also terpenes in the strict sense, but are considered separately due to their special biological importance. All terpenes have in common that they are built up from mevalonic acid and via the mevalonic acid route of the same name .
Terpenes
The terpene group of substances has a huge variety of carbon structures. What they all have in common, however, is that they are derived from isoprene and represent multiples of this molecule. One differentiates the terpenes according to the number of their carbon atoms. Practically all terpene structures have common names and are named after their biological source. Functional groups are often added as a prefix or suffix to the name of the carbon structure.
| Surname | Number of carbon atoms | Number of isoprene units | Examples |
|---|---|---|---|
| Isoprene | 5 | 1 | Isoprene |
| Monoterpenes | 10 | 2 | Menthol , carvone , thujanon , camphor |
| Sesquiterpenes | 15th | 3 | Farnesol , sesquisabines , cadalenol , artemisites |
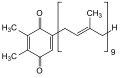
| Diterpenes | 20th | 4th | Retinal (Vitamin A), Paclitaxel , Rosan , Nimbion , Gibberellan |
| Sesterterpenes | 25th | 5 | Neomanoalid , Scalarin , Hyrial |
| Triterpenes | 30th | 6th | Squalene , Protostane , Lanosterol , Oleanane |
| Tetraterpenes | 40 | 8th | Carotenes (provitamin A), xanthophylls |
| Polyterpenes | > 40 | > 8 | Natural rubber , gutta-percha |
The monoterpenes also include the iridoids , which are characterized by the basic body iridodial .
The sesterterpenes represent a quantitatively small group of terpenes. If carbon atoms are removed during the metabolism, the compounds are given the prefix Nor, such as the sesquiterpene norpatchoulenol , in which a methane molecule has been formally removed from the original patchouli alcohol .
Terpenes fulfill a multitude of biological functions, ranging from aromas and fragrances to pheromones to vitamin functions (vitamin A) and hormone precursors (steroid hormones). Their technical applications range from pharmaceuticals ( taxol ) or steroids to insecticides ( pyrethroids ) and odorous substances for the cosmetics industry.
Steroids
Steroids are widespread natural substances in the animal and plant world. They are all derived from the triterpene squalene , which cyclizes to form the tetracyclic sterane structure. In naturally occurring steroids, rings B and C and rings C and D are each connected trans and these are called gonans . Rings A and B of the gonan can be connected either cis - (5β-gonan) or trans - (5α-gonan). With the natural steroids, these are always cis -connected, i.e. 5β-gonans. The most important steroid in humans and animals is cholesterol , which is not found in plants. Lipoproteins and steroid hormones are built from cholesterol , just like the hormones of the adrenal cortex ( corticosteroids ). The sex hormones of mammals, including humans, are steroids.
Polyprenyl hydroquinones
The human vitamin K as well as several other similar substances in all living things have the common feature of a hydroquinone residue and an attached polyprenyl residue. The best- known examples and their abbreviations are ubiquinones (in humans ubiquinone-10 , coenzyme Q-10), phylloquinone (vitamin K), menadione , menaquinone (MK), plastoquinone (PQ) and tocochinone . Relatively complex sample preparations and analytical methods are required for reliable detection of these substances , such as B. Tocoquinone. They act as electron transporters in the mitochondrial and bacterial respiratory chain .
Aromatic compounds
In organisms there are basically three biosynthetic pathways that lead to aromatic compounds - the shikimate path , the malonate path and the mevalonate path .
The shikimate path is based on the carbohydrate metabolism and runs through the shikimic acid to the aromatic natural substances. This path takes place mainly in higher plants. These natural substances are often characterized by highly oxidized phenolic aromatics with a linear side chain with functional groups. The phenolic groups are mostly in the 3-, 4- and 5-position of the side chain.
The malonate route (polyketide route) is based on fatty acid metabolism. The juxtaposition of acetate units creates a polycarbonyl compound which can cyclize to mono- or polynuclear aromatics in one or more aldol condensations . These compounds are characterized by highly oxidized aromatics, which often also contain quinones or hydroquinones , but without longer side chains. The oxygen groups are therefore in the 1,4-position. The malonate pathway is mainly found in microorganisms.

The Mevalonatweg also leads to aromatic natural substances, the terpenes. One example is the biosynthesis of thymol . The aromatics produced in this way often carry the isopropyl groups characteristic of terpenes .
Animal organisms seldom produce aromatic compounds themselves. These are therefore usually essential food components (aromatic amino acids and vitamins).
Phenylpropane derivatives
Phenylpropane derivatives are aromatic compounds with a propyl side chain. The aromatic often carries hydroxyl or methoxy groups. The propyl side chain can be either saturated or unsaturated, form a cycle or carry various functional groups. This class of compounds is formed in plants and microorganisms via the shikimate biosynthetic pathway . Along with terpenes, phenylpropanoids are the second most common constituent of essential oils. Well-known phenylpropanes are cinnamaldehyde , anethole and estragole . As a building material for wood, lignins are a polymer of the phenylpropane derivatives.
Flavonoids
Flavonoids belong to the plant pigments and are structurally derived from the phenylpropanoids. Therefore, one often finds phenols or methoxyphenols here . They are often glycosidically bound with carbohydrates and form the aglycon. Depending on the functional group on the heterocyclic ring, a distinction is made between flavan, flavone, flavonol, flavonone and flavonolol. Flavonoids are primarily important as plant pigments and form the majority of all flower pigments.
Tannins
The generic term tanning agents refers to inorganic and organic compounds that are able to convert animal hides into leather . The organic tanning agents have in common that they contain phenolic groups, but they do not form a uniform class of substances. The most important and best-known tanning agent, which is one of the natural substances, is tannin and is a polyhydroxyphenol. The tannins also include some phenolic flavones and their dimeric condensation products. These, like the flavones themselves, are often in the form of aglycones of glycosides.
Polyketides
The polyketides are a large and very heterogeneous group of natural products. It includes aliphatic , cyclic , acyclic and aromatic compounds. Their biological functions are often unknown. They have very large structural differences, but all belong to the same class of natural products. Polyketides are characterized by a common biosynthetic pathway. What they all have in common is that they contain a carbon backbone, which is made up of acetic acid and propionic acid. As with the terpenes, a distinction is made between the polyketides according to the number of acetate units.
| Surname | Number of acetates | Examples |
|---|---|---|
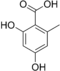
| Triketide | 3 |

|
| Tetraketides | 4th |
|
| Pentaketide | 5 |
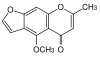
|
| Heptaketide | 7th |

|
| Polyketide alkaloids | Incorporation of ammonia |

|
| Anthraquinones | 8th |
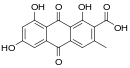
|
| Tetracyclines | 8 malonate units |

|
Polyketocarboxylic acids form the starting point for all ketides. Basically, we find the reactions in the biosynthesis of polyketides Claisen Esterkondensation , the aldol condensation and Dieckmann condensation .
The following reactions are known as aldol reactions , the formation of enol esters or ethers , methylations, chlorinations or hydroxylations, but also reductions of the carboxyl or carbonyl groups to alcohols or methylene groups. The decarboxylation of the β-keto acid group is also observed.
Heterocycles
Pteridines
The pteridines are derived from the basic structure of pteridine , for example the vitamins riboflavin (vitamin B 2 ) and folic acid , the coenzymes FAD and FMN as well as the molybdenum cofactors , which are all derived from molybdopterin . Their functions as cofactors are diverse.
Pyridine derivatives
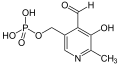
In particular nicotinic acid (vitamin B 3 ) and pyridoxal phosphate with its precursors pyridoxine , pyridoxal and pyridoxamine (vitamin B 6 ) fall into this substance class.
Nucleosides
As nucleosides are N -glycosides of heterocyclic designated systems. In a narrower sense, the building blocks of DNA and RNA are called nucleic acids. With these nucleic acids, the sugar residue is always a ribose (RNA) or deoxyribose (DNA). In the DNA are using deoxyribonucleic the genetic information stored. With the help of ribonucleic acids, the RNA can catalyze biochemical reactions and serve as a signal transmitter or information store.
Natural nucleosides with purine bases ( adenine , guanine ) and with pyrimidine bases ( cytosine , thymine and uracil ) are known.
| Purines | Pyrimidines | ||
|---|---|---|---|
 Adenine (A) |
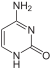 Cytosine (C) |
||
 Guanine (G) |
|
||
| Structural formulas of the nucleobases in DNA (A, G, C, T) and RNA (A, G, C, U). The N - glycosidic bond to ribose or deoxyribose in DNA takes place in each case at the NH group pointing downwards in the figures. | |||
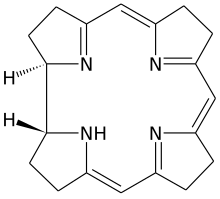
Porphyrins and corrinoids
The primary natural products also include a number of other classes of compounds, such as tetrapyrroles , which are formed from four pyrrole residues linked by a methine bridge. The ring-shaped tetrapyrroles, porphyrins and chlorins are of greatest importance here . The phorphyrins form the basis for chlorophyll , cytochrome and hemoglobin and are the complex ligand for an iron (II) atom. In the chlorophylls, the chlorins are the complex ligand for magnesium (II) as the central atom. They have a variety of tasks in the organism, ranging from oxygen transport and storage (hemoglobin and myoglobin ) to electron and energy transfer to catalysis of biochemical reactions (vitamin B 12 and cytochrome P 450) as a coenzyme .
Alkaloids
As early as 1806, the German pharmacist Friedrich Sertürner isolated morphine as the first alkaloid. The term alkaloids was coined in 1819 by Carl Friedrich Wilhelm Meißner , who understood it to mean all basic natural substances. The term was later expanded to include other nitrogen-containing natural substances. Today all nitrogen-containing natural substances are summarized under this name, even if there is still no uniform definition. Alkaloids often have biological effects and form an important basis as lead structures for active pharmaceutical ingredients.
There are various names for the alkaloid classes that are not used uniformly in the literature. On the one hand, alkaloids are named according to their botanical origin - Solanum -, Papaver -, Angostura -, Lobelia alkaloids, etc. - but on the other hand they are also divided into pyridine, quinoline or steroid alkaloids by their chemical parent compound.
Often only compounds are referred to as alkaloids which are derived from proteinogenic amino acids and contain aromatic nitrogen heterocycles. According to this definition, however, various nitrogen-containing natural substances such as coniine , piperine and caffeine are not alkaloids.
The systematic classification of the alkaloids is also not uniform. On the one hand there is the classification according to their chemical structure, i.e. according to the type of nitrogen heterocycle: There are then, for example, steroid , indole , pyridine or tropane alkaloids. The classification according to origin is also common: ergot alkaloids, curare or opiates .
In today's chemical literature, alkaloids are grouped into the following groups, which are classified according to their chemical structure:
- Alkaloids with a piperidine , pyrrole , pyrrolidine and pyridine skeleton
- Alkaloids with isoquinoline , quinoline , quinazoline and indole skeleton
- Alkaloids with indolizine , pyrrolizidine and quinolizidine scaffolds
- Purine alkaloids
- Steroid alkaloids
Biogenic amines
Biogenic amines are compounds that are formed by simply decarboxylating an amino acid and play a role as an important component of lipids, as a coenzyme or as a neurotransmitter ( acetylcholine , tryptamine , serotonin or histamine ). In pharmaceutical terms, L - dopa as a Parkinson's medication plays an important role here. Other well-known representatives are adrenaline , ephedrine and mescaline .
See also
literature
- Gerhard Habermehl, Peter E. Hammann, Hans C. Krebs, W. Ternes: Naturstoffchemie , 3rd, fully revised. and exp. Edition, Springer Verlag 2008, ISBN 978-3-540-73732-2 .
- Peter Nuhn : Natural Products Chemistry. Microbial, vegetable and animal natural products , 4th edition, S. Hirzel Verlag, Stuttgart 2006, ISBN 978-3-7776-1363-5 .
Web links
Individual evidence
- ↑ Peter Nuhn : Naturstoffchemie. Microbial, vegetable and animal natural products , 2nd edition, S. Hirzel Verlag, Stuttgart 1990, pp. 20-23; ISBN 3-7776-0473-9 .
- ^ Otto Krätz: 7000 Years of Chemistry , Nikol Verlagsgesellschaft, Hamburg 1999; ISBN 3-933203-20-1 .
- ↑ Explanation of terms and abbreviations. In: REACH Helpdesk. June 24, 2013. Retrieved July 29, 2019 .
- ↑ a b Regulation (EC) No. 1907/2006 on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in the consolidated version of July 2, 2019
- ↑ BMAS - Laws: Biological Agents Ordinance ( Memento from August 15, 2014 in the Internet Archive )
- ↑ A. Fleming: On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. In: Bull. World Health Organ . Volume 79, Number 8, 2001, pp. 780-790, PMID 11545337 . PMC 2566493 (free full text).
- ↑ Mansukhlal C. Wani, Harold Lawrence Taylor, Monroe E. Wall, Philip Coggon, Andrew T. McPhail: Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia , in: J. Am. Chem. Soc. , 1971, 93 , pp. 2325-2327; doi: 10.1021 / ja00738a045 .
- ↑ Gerhard Höfle, Norbert Bedorf, Heinrich Steinmetz, Dietmar Schomburg, Klaus Gerth, Hans Reichenbach: Epothilon A and B - novel, 16-membered macrolides with cytotoxic effects: isolation, structure in the crystal and conformation in solution , in: Angewandte Chemie , 1996, 108 , Pp. 1671-1673; doi: 10.1002 / anie.19961081342 .
- ↑ New introduction of zidovudine. In: Zeitschrift für Chemotherapie, 1987, Issue 4. Retrieved on August 4, 2013 .
- ↑ Lee Jia, Yuqing Zhao: Current Evaluation of the Millennium Phytomedicine - Ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and Regulations , in: Curr. Med. Chem. , 2009, 16 , pp. 2475-2484; PMC 2752963 (free full text).
- ↑ Lee Jia, Yuqing Zhao, Xing-Jie Liang: Current Evaluation of the Millennium Phytomedicine - Ginseng (II): Collected Chemical Entities, Modern Pharmacology, and Clinical Applications Emanated from Traditional Chinese Medicine , in: Curr. Med. Chem. , 2009, 16 , pp. 2924-2942; PMC 2754208 (free full text).
- ↑ Steven D. Ehrlich: Ginkgo Biloba Review. University of Maryland - Medical Center, December 13, 2010, accessed August 4, 2013 .
- ↑ S. Ganguli: Neem: A therapeutic for all seasons ( Memento of June 5, 2011 in the Internet Archive ) (PDF; 21 kB), in: Current Science , 2002, 82 , p. 1304 (Archive.org).
- ↑ Bernd Schäfer: Natural substances of the chemical industry , spectrum Akademischer Verlag, Heidelberg 2006; ISBN 978-3-8274-1614-8 .
- ^ Gareth Thomas: Medicinal Chemistry , 2nd Edition, John Wiley & Sons Ltd, West Sussex, 2007, pp. 90-110, 161-163; ISBN 978-0-470-02598-7 .
- ↑ Rolf Breinbauer, Ingrid R. Vetter, Herbert Waldmann: From protein domains to drug candidates - natural products as lead structures for the design and synthesis of substance libraries , in: Angewandte Chemie , 2002, 114 , pp. 3002–3015; doi : 10.1002 / 1521-3757 (20020816) 114: 16 <3002 :: AID-ANGE3002> 3.0.CO; 2-V .
- ↑ Herbert Waldmann: Naturally combinatorial - natural substance-driven drug development , in: Nachrichten aus der Chemie , 2003, 51 , pp. 126-131; doi: 10.1002 / nadc.20030510210 .
- ↑ Rudolf Hänsel, Otto Sticher (Ed.): Pharmakognosie - Phytopharmazie . 9th edition. Springer Verlag, Heidelberg 2009, ISBN 978-3-642-00962-4 , p. 18-29 .
- ↑ W. Kreis: Principles of secondary metabolism. (PDF) In: Phytochemical Basics. SWBplus catalog enrichment at the Baden-Württemberg Library Service Center , accessed on August 5, 2013 .
- ↑ Wolfgang Kraus, Michael Bokel, Adolf Klenk, Helmut Pöhn: The structure of azadirachtin and 22,23-dihydro-23β-methoxyazadirachtin , in: Tetrahedron Letters , 1985, 26 , pp. 6435-6438; doi: 10.1016 / S0040-4039 (00) 99020-8 .
- ↑ G. Büchi, RE Erickson, N. Wakabayashi: Terpenes. XVI.1,2 Constitution of Patchouli Alcohol and Absolute Configuration of Cedrene , in: J. Am. Chem. Soc. , 1961, 83 , pp. 927-938; doi: 10.1021 / ja01465a042 .
- ↑ G. Büchi, William D. Macleod: Synthesis of Patchouli Alcohol , in: J. Am. Chem. Soc. , 1962, 84 , pp. 3205-3206; doi: 10.1021 / ja00875a047 .
- ↑ Gareth Thomas: Medicinal Chemistry pp. 90-110.
- ↑ Peter Bützer: Article on vitamin B 12 . (PDF; 481 kB) (No longer available online.) In: Molecular Dynamics - System Dynamics . January 2008, archived from the original on December 6, 2012 ; Retrieved August 4, 2013 .
- ^ KC Nicolaou, EJ Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , VCH Verlagsgesellschaft mbH, Weinheim, 1996, pp. 711-729; ISBN 3-527-29284-5 .
- ^ KC Nicolaou, EJ Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , pp. 655-671.
- ^ The Nobel Prize in Chemistry 1981. In: Nobelprize.org. Retrieved August 4, 2013 .
- ^ The Nobel Prize in Chemistry 1965. In: Nobelprize.org. Retrieved August 4, 2013 .
- ^ KC Nicolaou, EJ Sorensen: Classics in Total Synthesis: Targets, Strategies, Methods , pp. 99-134.
- ^ KC Nicolaou, SA Snyder: Classics in Total Synthesis II , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003; ISBN 978-3-527-30684-8 .
- ↑ Stefan Abrecht, Peter Harrington, Hans Iding, Martin Karpf, René Trussardi, Beat Wirz, Ulrich Zutter: The Synthetic Development of the Anti-Influenza Neuraminidase Inhibitor Oseltamivir Phosphate (Tamiflu ® ): A Challenge for Synthesis & Process Research , in: CHIMIA International Journal for Chemistry , 2004, 58 , pp. 621-629.
- ↑ R. Carle: Phytochemicals - antibodies and nutraceuticals? In: vetline.de. October 10, 2007, accessed August 5, 2013 . (PDF).
- ^ Bernhard Watzl, Claus Leitzmann: Bioactive substances in food , 3rd edition, Hippokrates Verlag GmbH Stuttgart, 2005, p. 15; ISBN 3-8304-5308-6 .
- ↑ Peter Nuhn: Naturstoffchemie , p. 23.
- ^ Hans Beyer , Wolfgang Walter : Textbook of organic chemistry , 21st edition, S. Hirzel Verlag, Stuttgart 1988, pp. 822-828; ISBN 3-7776-0438-0 .
- ↑ Hans-Dieter Belitz , Walter Grosch: Textbook of food chemistry . 4th edition. Springer Verlag, Heidelberg / Berlin 1992, ISBN 3-540-55449-1 , p. 9 .
- ↑ Peter Nuhn: Naturstoffchemie , p. 77.
- ↑ Peter Nuhn: Naturstoffchemie , pp. 159-160.
- ^ Gerhard Michal: Biochemical Pathway , Spectrum Akademische Verlagsgesellschaft, Heidelberg 1999, pp. 37–40; ISBN 3-86025-239-9 .
- ↑ Beyer, Walter: Textbook of Organic Chemistry , p. 405.
- ↑ Peter Nuhn: Naturstoffchemie , pp. 200–201.
- ↑ Peter Nuhn: Naturstoffchemie , p. 209.
- ↑ Peter Nuhn: Naturstoffchemie , pp. 174-181.
- ^ Gerhard Habermehl, Peter E. Hammann, Hans C. Krebs, Naturstoffchemie , 2nd edition, Springer Verlag, 2002, p. 385; ISBN 3-540-43952-8 .
- ↑ Peter Nuhn: Naturstoffchemie , p. 297.
- ^ Nomenclature of Glycolipids. In: IUPAC . Retrieved January 28, 2016 .
- ↑ Peter Nuhn: Naturstoffchemie , p. 397.
- ^ Roger RC New (Editor), Liposomes a practical approach , IRL Press at Oxford University Press, Oxford 1990, p. 13; ISBN 0-19-963077-1 .
- ↑ William H. Elliott, Daphne C. Elliott: Biochemistry and Molecular Biology , forth ed., Oxford University Press, Oxford 2009, pp. 16-26; ISBN 978-0-19-922671-9 .
- ↑ Peter Nuhn: Naturstoffchemie , pp. 311–322.
- ↑ Leopold Ružička: Nobel Lecture: Multimembered Rings, Higher Terpene Compounds and Male Sex Hormones. In: Nobelprize.org. December 12, 1945, accessed August 6, 2013 . (PDF; 525 kB)
- ↑ Katharina Munk: Basic studies in biology: botany . Spectrum Academic Publishing House, Heidelberg 2001; ISBN 3-8274-0909-8 .
- ↑ Eberhard Breitmeier: Terpenes: Aromen, Düfte, Pharmaka, Pheromone , BG Teubner, Stuttgart 1999; ISBN 3-519-03548-0 .
- ↑ Beyer, Walter: Textbook of Organic Chemistry , pp. 678–679, 686–698.
- ↑ Melchert HU, Pollok D, Pabel E, Rubach K, Stan HJ: Determination of tocopherols, tocopherolquinones and tocopherolhydroquinones by gas chromatography-mass spectrometry and preseparation with lipophilic gel chromatography. , J Chromatogr A. 2002 Nov 8; 976 (1-2): 215-20, PMID 12462612
- ^ Gerhard Michal: Biochemical Pathway , Spektrum Akademische Verlagsgesellschaft, Heidelberg, 1999, pp. 59–60, 85–86 and 192; ISBN 3-86025-239-9 .
- ↑ Mikio Yamazaki, Taeko Usui, Shoji Shibata: The Biogenesis of Plant Products. II. The Biogenesis of Thymol. ; in: Chemical & Pharmaceutical Bulletin , 1963, 11 , pp. 363-365; doi: 10.1248 / cpb.11.363 (full text).
- ↑ Peter Nuhn: Naturstoffchemie , p. 522.
- ↑ Peter von Sengbusch, Paul von Sengbusch: Phenolic substances. In: The secondary metabolism of plants. Secondary plant products (plant substances). Botany online 1996-2004 from the University of Hamburg , accessed on August 6, 2013 .
- ↑ Otto Th. Schmidt, Walter Mayer: Natural tannins , in: Angewandte Chemie , 1956, 68 (3), pp. 103-115; doi: 10.1002 / anie.19560680305 .
- ↑ Beyer, Walter: Textbook of Organic Chemistry , pp. 801–803, 882–883.
- ↑ Beyer, Walter: Textbook of Organic Chemistry , pp. 762, 768–769.
- ↑ Beyer, Walter: Textbook of Organic Chemistry , pp. 851–858.
- ↑ Beyer, Walter: Textbook of Organic Chemistry , pp. 710–717.
- ↑ Gerhard Habermehl, Peter E. Hammann, Hans C. Krebs: Naturstoffchemie , pp. 131–243.
- ↑ Peter Nuhn: Naturstoffchemie , pp. 553–597.