Dieckmann condensation
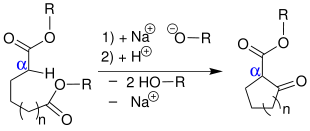
The Dieckmann condensation is a name reaction of organic chemistry and named after the German chemist Walter Dieckmann (1869–1925). In chemistry, this reaction is understood as an intramolecular Claisen condensation of dicarboxylic acid esters to form cyclic β - keto esters .
Reaction mechanism
The mechanism is identical to that of the Claisen condensation , but the reaction is only complete with a stoichiometric amount of base , which is present in the form of alcoholates , sodium amides or hydrides of the alkali metals . The base causes a deprotonation of the dicarboxylic acid ester 1 . The next step is a nucleophilic attack on the carbonyl carbon. An alcoholate 4 is split off with formation of the cyclic β-keto ester 5 . Then 5 is deprotonated by a further alcoholate in the α-position to both carbonyl groups. The deprotonation of 5 has a high driving force and is practically irreversible. So the balance can be shifted to the side of the desired product. When the reaction is complete, acid is added to the batch in order to neutralize the anion , whereby all alcohol ions are also protonated and a reverse reaction is excluded. The products of a Dieckmann condensation are five- to eight-membered, cyclic β-ketoesters. Under certain conditions, rings with twelve carbon atoms or even larger can be synthesized. The production of 9- to 13-membered rings is difficult due to transannular stresses , so the yields for these ring sizes are very low, even when working according to the Ziegler-Ruggli dilution principle .
See also
literature
- R. Brückner: reaction mechanisms . 3rd edition, 2003, ISBN 978-0-12-429785-2 , p. 572 f.
Individual evidence
- ↑ W. Dieckmann: On the knowledge of ring formation from carbon chains. In: Reports of the German Chemical Society. 27, No. 1, 1894, pp. 102-103, doi: 10.1002 / cber.18940270126 .
- ↑ W. Dieckmann: About a ring-shaped analogue of the ketipic acid ester. In: Reports of the German Chemical Society. 27, No. 1, 1894, pp. 965-966, doi: 10.1002 / cber.189402701202 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Amsterdam 2005, ISBN 978-0-12-369483-6 , pp. 138-139.
- ^ Siegfried Hauptmann : Organic chemistry . 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, ISBN 3-342-00280-8 , p. 454.
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry. S. Hirzel, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 279.