Disaccharides
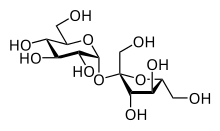
Disaccharides (outdated double sugars , rarely biosens ) are organic-chemical compounds from the group of carbohydrates . Formally, disaccharides are formed by splitting off water between two monosaccharides . The two monosaccharides ( simple sugars ) are covalently linked in the disaccharide via a glycosidic bond . Disaccharides belong to the group of oligosaccharides .
The economically most important disaccharide is cane and beet sugar, sucrose. Today it is obtained industrially from sugar cane and sugar beet and does not represent an essential part of human nutrition . What all disaccharides have in common is their sweet taste, and sucrose in particular is used today as a sweetener in human nutrition.
nomenclature
The numbering of the carbohydrates begins at the carbon end with the most highly oxidized carbon atom, which in the open-chain form of the monosaccharides carries an aldehyde residue or is adjacent to a ketone . The designations α and β define the stereochemistry at the anomeric center .
An abbreviated notation uses a three-letter abbreviation for the monosaccharides and the suffixes f and p for furanose and pyranose .
properties
Disaccharides are colorless solids that are easily soluble in water , sparingly soluble in ethanol and insoluble in most organic solvents. Both α- and β-linked disaccharides are known, as well as links at every position. The 1,4- and 1,6-linked disaccharides are by far the most abundant in nature. Like monosaccharides, disaccharides are temperature-sensitive and decompose when heated to temperatures above their melting point or even at the melting point and therefore cannot be distilled in a vacuum. Purification is therefore normally carried out by recrystallization or, in small amounts, by chromatography . In solution, disaccharides are mostly stable at pH values ≥7, but hydrolyze even in the weakly acidic range. The acid hydrolysis of disaccharides produces two monosaccharides, for example, sucrose ( empirical formula C 12 H 22 O 11 ) produces the monosaccharides D -glucose (C 6 H 12 O 6 ) and D- fructose (empirical formula C 6 H 12 O 6 ) . The best-known and most common disaccharide is sucrose, household sugar, cane and beet sugar.
Depending on how the two simple sugars are connected, a distinction is made between reducing and non- reducing disaccharides. In the case of the reducing disaccharides, at least one anomeric carbon atom is not involved in the glycosidic bond. In the case of the non-reducing disaccharides, the two anomeric carbon atoms are directly linked. Reducing double sugars are stable to hydrolysis in the basic pH range , but are very sensitive to oxidizing agents .
In the crystalline state and in aqueous solution, many disaccharides such as cellobiose, lactose and sucrose are stabilized by intramolecular hydrogen bonds (HBB), which also determines the most stable conformation of the molecule. The HBB can also vary for non-aqueous solvents.
Occurrence

Disaccharides are rarely found in animal organisms. The exceptions are the trehalose in the hemolymph of most insects and the lactose in the milk of mammals . Disaccharides are very common in plants . Sucrose is found in fruit and many fruit and vegetable juices ; from sugar cane or sugar beet it is used as table sugar recovered. Trehalose is also found in yeast and mushrooms, as well as algae . Maltose is not found in free form, but is produced during the enzymatic breakdown of starch , for example during digestion . A well-known breakdown product is invert sugar , which is the main component of honey and consists in equal parts of fructose and glucose and is formed from sucrose (e.g. in flower nectar).
Common disaccharides
The two monosaccharide units of the disaccharide can also be connected to one another at different points and have different stereochemistry at the connection point . This creates a multitude of disaccharides.
| Surname | chemical compound | Occurrence |
|---|---|---|
| Cellobiose | Glucose -β- (1 → 4) -glucose | Cellulose disaccharide |
| Gentiobiosis | Glucose-β- (1 → 6) -glucose | Glycosides ( amygdalin ) |
| Isomaltose | Glucose-α- (1 → 6) -glucose | Branches in glycogen and starch |
| Isomaltulose | Glucose-α- (1 → 6) - fructose | enzymatic extraction from sucrose |
| Lactose | Galactose -β- (1 → 4) -glucose | Lactose |
| Lactulose | Galactose-β- (1 → 4) -fructose | Reversion product of lactose |
| Laminaribiosis | Glucose-β- (1 → 3) -glucose | Laminarin degradation product is also produced when brewing beer |
| Maltose | Glucose-α- (1 → 4) -glucose | Malt sugar, or disaccharide of starch , in sugar beet, bee honey |
| Maltulose | Glucose-α (1 → 4) -fructose | Reversion product of maltose |
| Melibiosis | Galactose (1 → 6) glucose | in cocoa bean |
| Neohesperidosis | Rhamnose - (1 → 2) -glucose | in glycosides ( naringin , neohesperidin ) |
| Neotrehalosis | Glucose- (1 → 1) -glucose | in koji extract ( Aspergillus oryzae ) |
| Nigerose | Glucose (1 → 3) glucose | in honey, beer |
| Rutinosis | Rhamnose (1 → 6) glucose | in anthocyanins , glycosides ( hesperidin ) |
| Sambubiosis | Xylose-β- (1 → 2) -glucose | in anthocyanins |
| Sophorosis | Glucose (1 → 2) glucose | in anthocyanins , in legumes |
| Sucrose | Glucose-α- (1 → 2) -fructose | Cane or beet sugar |
| Trehalose | Glucose-α, α '- (1 → 1) -glucose | in the hemolymph of lower animals, ergot , young mushrooms |
Enzymatic degradation
Disaccharides found in food cannot enter glycolysis directly , but must first be hydrolyzed extracellularly to monosaccharides . The corresponding degradation enzymes from the group of glycosidases are called "disaccharidases". In humans and many mammals, they are located in the microvilli of the mucous membrane of the small intestine . Examples of such hydrolases are the maltases ( lysosomal α-glucosidase and maltase-glucoamylase ), lactase , β-galactosidase , invertase (also known as “saccharase”) and trehalase .
literature
- Belitz , Grosch, Schieberle : Textbook of food chemistry. 6th edition. Springer, 2007, ISBN 978-3-540-73201-3 , doi : 10.1007 / 978-3-540-73202-0 .
- Römpp - food chemistry. 9th edition, Thieme, Stuttgart 1995; Pp. 272, 273; ISBN 3-13-736601-1 .
Web links
Individual evidence
- ↑ German-English Dictionary: Zweifachzucker (Disaccharide)
- ↑ Entry on biosen. In: Römpp Online . Georg Thieme Verlag, accessed on February 26, 2013.
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th edition, de Gruyter Verlag, Berlin, 2011, pp. 774-776, ISBN 978-3-11-024894-4 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 649, ISBN 3-342-00280-8 .
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th edition, de Gruyter Verlag, Berlin, 2011, p. 775, ISBN 978-3-11-024894-4 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules . Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 340.