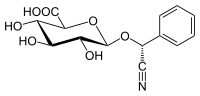
Amygdalin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| D- Amygdalin | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Amygdalin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 27 NO 11 | |||||||||||||||
| Brief description |
colorless trihydrate orthorhombic crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 457.4 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.4 g cm −3 |
|||||||||||||||
| Melting point |
223-226 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Amygdalin (Greek amygdalis , almond kernel) is a cyanogenic glycoside that splits off in the presence of water and the enzyme mixture emulsin hydrocyanic acid (HCN).
Chemical properties
In dilute acids, amygdalin is split into gentiobiosis and mandelonitrile (the nitrile of mandelic acid ). The latter breaks down further into the typical bitter almond flavors benzaldehyde and hydrogen cyanide; the gentiobiosis is hydrolyzed to two glucose molecules .
Stone fruit contains the enzyme mixture emulsin, which consists of β-glucosidases and hydroxynitrile lyase ; this mixture also splits amygdalin in a multi-stage reaction to form two molecules of glucose, benzaldehyde and hydrocyanic acid. Here, amygdalin is first hydrolysed to prunasin and D - glucose by a β-glucosidase amygdalin hydrolase ( EC 3.2.1.117 ) , and then the glucose portion of prunasin is split off by a prunasin hydrolase ( EC 3.2.1.21 ), resulting in mandelonitrile. Mandelonitrile can spontaneously decompose to benzaldehyde and HCN (in a neutral or alkaline medium), a mandelonitrile lyase (also known as hydroxynitrile lyase, EC 4.1.2.10 ) catalyzes this reaction considerably.
One gram of amygdalin produces 59 mg of HCN.
Stereoisomers
The L - epimer , also S-stereoisomer ( L -mandelonitrile- β - D -gentiobioside) is called neoamygdalin . Amygdalin and neoamygdalin are diastereomers , but not enantiomers , since the configurations of the ten stereogenic centers of gentiobiosis do not change.
Neoamygdalin and amygdalin can be separated by chiral chromatography.
Occurrence

Bitter apricot kernels , apple kernels , bitter almonds and seeds from other stone fruits such as B. Plums contain amygdalin in high concentrations. This can be broken down into hydrogen cyanide, benzaldehyde and glucose when the oil seeds are processed , for example into Persipan . The released hydrocyanic acid must be removed, the kernels are "debittered".
In 1830 amygdalin was isolated from bitter almonds.
Occurrence in food
Amygdalin, prunasin and other cyanogenic glycosides ( linamarin , lotus tralin (flax, legumes , manioc, etc.), dhurrin (millet), taxiphyllin (bamboo shoots), sambunigrin ( elderberry ) and over 70 others) come in relevant quantities in some unprocessed foods (> 0.02% bound hydrocyanic acid). However, boiling reduces the hydrogen cyanide content to harmless concentrations.
The stone fruits of some rose plants have the highest levels of hydrocyanic acid , v. a. Bitter almonds and apricot kernels. Apricot kernels contain up to 8% amygdalin, corresponding to around 0.4% bound hydrogen cyanide, bitter almonds up to 5% amygdalin (corresponding to 0.3% hydrocyanic acid).
Amygdalin supporters often mention other foods that either contain only insignificant amounts of cyanogenic glycosides ( blackberries , strawberries, haricot beans , peas ) or where the hydrogen cyanide is largely removed by cooking (cassava / tapioca , yams , lima beans ).
The raw lima bean contains, for example, 0.2-0.3% bound hydrogen cyanide (200-300 mg / 100 g), haricot beans and peas only 0.002% (2 mg / 100 g), cherry juice still 0.00005% ( 500 µg / l). A single apricot kernel already contains 0.5 mg hydrogen cyanide, so it is better not to consume more than one or two such kernels per day. The lethal dose in humans is around 50 mg hydrogen cyanide (0.5-3.5 mg / kg body weight); hydrogen cyanide is only slowly broken down by the enzyme rhodanase to form rhodanide . Therefore about 40 kernels in one hour are fatal in an adult weighing 60 kg.
On the other hand, 5 µg / kg of body weight are considered harmless, as they are never exceeded by normal foods, especially since foods with a slightly higher content of bound hydrogen cyanide (e.g. legumes) are usually consumed cooked. However, it should be noted that hydrogen cyanide accumulates in the body as it is difficult to break down. Regular intake of sublethal doses (e.g. regular intake of apricot kernels) can therefore lead to hydrogen cyanide poisoning in the long term.
biosynthesis
In plants, amygdalin is synthesized from L - phenylalanine . Here, phenylalanine is enzymatically converted with the aid of cytochrome P450 to phenylacetaloxime with elimination of CO 2 , which is then converted into phenylacetonitrile with elimination of water . A Cyp71 enzyme then hydroxylates it to mandelonitrile, followed by glycosylation using UDP-glucose to form prunasin. Another glucose molecule is attached to its 6-hydroxyl group, which ultimately creates amygdalin.
Use in alternative medicine
Amygdalin ( "Amigdalina") is, as well as the semi-synthetic and also cyanogenic Lae vo -Mandelsäureni tril -β-glucuronide ( "Laetrile", "Laetrile"), under the fantasy name vitamin B 17 , an alternative medicine for the prevention and treatment of tumor diseases ( Cancer ), especially used in the US from the 1970s and 80s. The designation as a vitamin is misleading, however, since amygdalin is not an essential substance for the human metabolism . In addition, amygdalin has no nutritional properties.
In cancer treatment, oral (“B17” tablets, chewing apricot kernels ) and intravenous administration of amygdalin is described. From the point of view of scientifically based medicine, amygdalin is to be regarded as a "dubious miracle drug" in this application. The mechanism of action claimed by supporters of alternative medicine is said to be based on a breakdown of amygdalin into benzaldehyde, glucose and the highly toxic hydrogen cyanide with the participation of the enzyme β-glucosidase . An allegedly increased occurrence of β-glucosidase in tumor cells would locally produce more toxic hydrogen cyanide and lead selectively to the death of the tumor cell. In fact, however, β-glucosidase occurs in largely the same and only extremely small amounts in healthy cells and in tumor cells, as was already shown in the 1980s. Another hypothesis is that the alleged lack of the enzyme rhodanase in tumor cells selectively causes hydrogen cyanide to accumulate there. Rhodanase is able to detoxify small amounts of cyanides by converting them into the comparatively non-toxic thiocyanate . However, cancer cells also have a similar amount of rhodanase as normal cells. It was also alleged that amygdalin is hydrolyzed to mandelonitrile after ingestion , and that this is converted into a β-glucuronide after transport in the liver; the β-glucuronide should then be transported to the cancer cells and then release mandelonitrile and finally hydrogen cyanide. This is wrong, however, because mandelonitrile spontaneously dissociates to benzaldehyde and hydrogen cyanide beforehand, and it cannot be stabilized in the body by glycosylation .
According to clinical studies, amygdalin is almost completely excreted in the urine after injection. Orally administered amygdalin, however, sometimes leads to high and critical hydrocyanic acid concentrations in the body. The claim that cyanides are released especially in cancer cells and that they lead to a selective therapeutic effect there has been refuted by studies. On the other hand, there are attempts to increase the selective effect through drug targeting by binding β-glucosidase to cancer-associated monoclonal antibodies .
There is no scientifically based evidence of therapeutic effectiveness. In the USA, the FDA has been warning against ingestion for decades; it is considered a "quack drug". A clinical study from 1982 with 178 patients showed no therapeutic effect (cure, stabilization or amelioration of the disease, prolongation of life) after taking amygdalin in people suffering from cancer, but symptoms of cyanide poisoning did occur in some patients . A review from 2006 found that there are no clinical studies that provide evidence of therapeutic efficacy. A Cochrane review from 2011 (or the update from 2015) also came to this conclusion, the negative benefit-harm balance was confirmed. On the other hand, numerous symptoms of poisoning and dozens of deaths due to the use or erroneous ingestion by children have been described. Amygdalin has a clear toxicological potential. Use outside of clinical trials - and there only with very high safety precautions and the closest monitoring - is not advocated. The toxicity of amygdalin is further increased by (high-dose) vitamin C intake. This is because vitamin C increases the formation of cyanide from amygdalin and at the same time lowers the supplies of cysteine necessary for detoxification .
- Legal aspects for Germany
Doctors have a free choice of drugs within the scope of therapy freedom. This is restricted solely by Section 5 of the German Medicines Act , which prohibits the dispensing and use of questionable medicines. There are no finished medicinal products containing amygdalin on the market. The extent to which amygdalin or other custom-made products containing mandelonitrile ( prescription drugs ) are to be regarded as questionable can vary from case to case. Officially, they are generally classified as questionable because there is a reasonable suspicion of a release of toxic hydrogen cyanide. Nevertheless, in 2007, after a legal dispute, the marketability of the amygdalin-containing prescription drug prepared there on a medical prescription was finally granted to a pharmacy in Hanover. The court ruling was based on a toxicological report, according to which the formation of hydrogen cyanide in the present prescription drug was to be ruled out and this therefore did not pose a health risk. The starting material used is highly pure amygdalin, which, due to the lack of contamination with amygdalase, does not suggest any enzymatic cleavage with the result of hydrogen cyanide formation. The court, however, denied the general harmlessness of amygdalin-containing prescription drugs and referred to the concurring opinions of the Chamber of Pharmacists and court experts who warned against taking amygdalin of unclear origin and unclear purity. The official assessment of the dubiousness of drugs containing amygdalin was reaffirmed in 2014 by the Federal Institute for Drugs and Medical Devices (BfArM).
unwanted effects
After ingesting amygdalin-containing foods or preparations, there is a risk of fatal poisoning by hydrogen cyanide . Fatal cases of poisoning from apricot kernels are well documented in the toxicological literature. In regions where the consumption of apricot kernel preparations is common, the amygdalin content is reduced by the preparation technique. If these preparation techniques are not strictly followed, fatal cases of poisoning can occur. In the toxicological literature, cases of poisoning are also specifically described for therapy with amygdalin.
Amygdalin causes headache, dizziness, nausea and vomiting, if the level is high (> 3 μg / ml) there is a danger to life. If taken with foods high in beta-glucosidase, such as apricot kernels, the risk of toxicity is increased. This is further increased by taking supplements or foods with a high vitamin C content.
The lowest lethal dose for an adult weighing 60 kg is 0.57 mg / kg body weight, which is around 40 apricot kernels. If one considers the hydrogen cyanide content against the background of the lowest value of the metabolism rate (detoxification rate) for hydrogen cyanide of 0.1 mg / kg / h, the following figures result: An adult can use it to detoxify 6.0 mg hydrogen cyanide per hour by metabolizing, which is a consumption rate of around 7 cores per hour. The lethal dose determined for rats is between 405 mg / kg (pure substance), 522 mg / kg (pure substance), below 600 mg / kg (with glucosidase) and 880 mg / kg (pure substance).
literature
- C. Campa et al .: Analysis of cyanogenic glycosides by micellar capillary electrophoresis. In. J. Chromatogr. B Biomed. Sci. Appl. 739 (1), 2000, pp. 95-100, PMID 10744317 .
Web links
- Two bitter apricot kernels per day are the limit for adults - children should avoid them, BfR updated opinion no. 009/2015 of 7 April 2015 (PDF file; 35 kB).
- Independent specialist information of the CAM Cancer Project of the European Commission : CAM Summary Laetrile , revised version of December 13, 2011.
- National Cancer Institute: Laetrile / Amygdalin in the Physician Data Query (PDQ) .
- Ralf Nowotny: Has a cancer-curing vitamin been banned? In: mimikama . November 3, 2017, accessed January 15, 2020 .
Individual evidence
- ↑ a b c Datasheet Amygdalin (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b c d Entry on amygdalin. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b c Entry on Amydaglin in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c Entry on amygdalin in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 9, 2016.
- ↑ Emulsin. In: Spektrum.de . 1999, accessed April 10, 2020 .
- ↑ a b c d e Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels . In: EFSA Journal . 14, No. 4, 2016, ISSN 1831-4732 . doi : 10.2903 / j.efsa.2016.4424 .
- ↑ Albert Gossauer : Structure and reactivity of biomolecules. Helvetica Chimica Acta , Zurich, 2006, ISBN 978-3-906390-29-1 , p. 458.
- ↑ a b c d e f Klaus Pietrzik, Ines Golly, Dieter Loew: Handbook Vitamins: for prophylaxis, advice and therapy . 1st edition. Elsevier, Urban & FischerVerlag, Munich 2008, ISBN 978-3-437-55361-5 , pp. 459 .
- ↑ a b Stone fruit (Prunoidaea) on gift Pflanzen.com.
- ↑ Bitter almond (Prunus dulcis var. Amara) on gift Pflanzen.com.
- ↑ H.-D. Belitz, W. Grosch, P. Schieberle: Textbook of food chemistry. 6th edition, 2008, Springer-Verlag Berlin / Heidelberg, ISBN 978-3540732013 .
- ↑ a b Ralf Nowotny: Was a cancer-curing vitamin banned? In: mimikama . November 3, 2017, accessed on January 15, 2020 (German).
- ↑ a b Statement amygdalin. (PDF) In: Prevention and Integrative Oncology (PRiO). German Cancer Society , April 24, 2017, accessed January 15, 2020 .
- ↑ NN: unproven Methods of Cancer Management. Laetrile. In: CA Cancer J. Clin. Vol. 41, 1991, pp. 187-192; PMID 1902140 , PDF ( Memento of March 16, 2007 in the Internet Archive ).
- ↑ a b c d e f Questionable "cancer drug" Amygdalin / Laetrile - no end in sight . ( arznei-telegramm.de [accessed on October 5, 2018]).
- ↑ James A. Duke: CRC Handbook of Medicinal Spices . 1st edition. Taylor & Francis Inc., 2003, ISBN 978-0-8493-1279-3 , pp. 261-262 .
- ↑ CG Moertel et al .: A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. In: N. Engl. J. Med. 306 (4), 1982, pp. 201-206, PMID 7033783 .
- ↑ Thilo Bertsche, Martin Schulz: Amygdalin - a new old cancer drug? In: Pharmaceutical newspaper . April 24, 2003, online , accessed November 2, 2016.
- ^ Stefania Milazzo, Stephane Lejeune and Edzard Ernst : Laetrile for cancer: a systematic review of the clinical evidence . In: Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer . tape 15 , no. 6 , June 2007, p. 583-595 , doi : 10.1007 / s00520-006-0168-9 , PMID 17106659 .
- ^ Stefania Milazzo et al .: Laetrile treatment for cancer . In: The Cochrane Database of Systematic Reviews . No. 11 , November 9, 2011, p. CD005476 , doi : 10.1002 / 14651858.CD005476.pub3 , PMID 22071824 .
- ↑ a b Stefania Milazzo and Markus Horneber: Laetrile treatment for cancer . In: The Cochrane Database of Systematic Reviews . No. 4 , April 28, 2015, p. CD005476 , doi : 10.1002 / 14651858.CD005476.pub4 , PMID 25918920 , PMC 6513327 (free full text).
- ↑ AMK recommendations for testing the dispensability of prescription drugs (PDF; 423 kB), April 2011.
- ^ Judgment of the Lower Saxony Higher Administrative Court (AZ 11 LB 350/05) on an amygdalin prescription drug from Hanover PDF .
- ↑ N. Lilienthal: Amygdalin - lack of effectiveness and harmful side effects . In: Bulletin on drug safety . No. 3, 2014, pp. 7-13.
- ^ V. Herbert: Laetrile: the cult of cyanide. Promoting poison for profit. In: Am. J. Clin. Nutr. 32 (5), 1979, pp. 1121-1158, PMID 219680 , PDF .
- ↑ E. Lindner: Toxicology of Food. 4th edition, Wissenschaftliche Verlagsgesellschaft, 1990, ISBN 978-3-8047-1575-2 .
- ^ WA Kaschuba: Bavarian State Office for Health and Food Safety.
- ↑ GW Newton, ES Schmidt, JP Lewis et al .: Amygdalin toxicity studies in rats predict chronic cyanide poisoning in humans. In: The Western journal of medicine. Volume 134, Number 2, 1981, pp. 97-103, PMID 7222669 , PMC 1272529 (free full text).
- ↑ a b S. R. Adewusi, OL Oke: On the metabolism of amygdalin. 1. The LD50 and biochemical changes in rats. In: Canadian Journal of Physiology and Pharmacology . Volume 63, Number 9, 1985, pp. 1080-1083, PMID 2932206 .