Rhodanase
| Rhodanase | ||
|---|---|---|
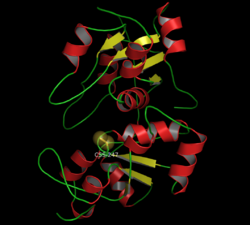
|
||
| Ribbon model of bovine rhodanase with persulfided cysteine residue C247 in the catalytic center (as a dome) according to PDB 1BOH | ||
| Properties of human protein | ||
| Mass / length primary structure | 296 amino acids | |
| Identifier | ||
| Gene name | TST | |
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 2.8.1.1 , transferase | |
| Response type | Transfer of a sulfur atom | |
| Substrate | Cyanide, iron, sulfite, persulfide | |
| Products | Thiosulfate, thiocyanate, sulfur / iron clusters | |
As rhodanese , also sulfur transferase, are enzymes referred to the sulfur of thiosulfate transmitted or persulfide to other molecules. The transfer of persulfide is an important part of sulfide oxidation , thus the detoxification of the constant when Cysteinabbau resulting hydrogen sulfide . With the transfer of sulfur to cyanides , these are also detoxified into thiocyanates in the body . The transfer to iron produces the iron-sulfur clusters that function as a cofactor . Rhodanases can be found in many bacteria and fungi. The rhodanases of the multicellular cells are located in the mitochondria or the chloroplasts . The human TST also shows weak mercaptopyruvate sulfur transferase activity.
Rhodanases belong to the sulfur transferases .
Catalyzed reaction
The reaction takes place in two steps.
In the first step, the disulfidic bond is formed in the catalytic center of the enzyme by the sulfur donor transferring its sulfur atom to the thiol group in the cysteine residue C247, forming a disulfane .
In the second step, the substrate is sulfided with regression of the thiol group .
The main substrate is the persulfide of sulfide: quinone oxidoreductase, which gives off the sulfur that is transferred to sulfite in the second step, which becomes thiosulfate.
In the less important cyanide sulphuration, thiosulphate is the donor and cyanide is the acceptor of the sulfur atom:
The effectiveness of thiosulfate (e.g. in the form of a sodium thiosulfate solution ) in cyanide poisoning is also based on the activation of this enzymatic detoxification system.
Rhodanase as a domain
Amino acids 25-143 and 173-288 (starting Met as 1) each form a catalytic protein domain , which is also found in several other proteins, such as mercaptopyruvate sulfur transferase and M-phase inducer phosphatases .
literature
- F. Gliubich, M. Gazerro, G. Zanotti, S. Delbono, G. Bombieri, R. Berni: Active Site Structural Features for Chemically Modified Forms of Rhodanese . In: Journal of Biological Chemistry . tape 271 , no. 35 , 1996, pp. 21054-21061 . PMID 8702871 PDF
- R. Cipollone, P. Ascenzi, P. Tomao, F. Imperi, P. Visca: Enzymatic detoxification of cyanide: clues from Pseudomonas aeruginosa Rhodanese. In: Journal of Molecular and Microbiological Biotechnology . Vol. 15, No. 2,3, 2008, pp. 199-211. PMID 18685272
Individual evidence
- ↑ UniProt Q16762
- ↑ a b T. M. Hildebrandt, MK Grieshaber: Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. In: The FEBS Journal Volume 275, Number 13, July 2008, pp. 3352-3361. doi : 10.1111 / j.1742-4658.2008.06482.x . PMID 18494801 .




