Monoclonal antibody
Monoclonal antibodies are antibodies , i.e. immunologically active proteins that are produced by a cell line (cell clone), which can be traced back to a single B lymphocyte and which are directed against a single epitope . A physiologically (naturally) occurring immune response against an antigen that has penetrated the body is, however, always polyclonal and is directed e.g. B. against many different epitopes on a bacterium.
Monoclonal antibodies play an important role in diagnostics and research, as they can bind a number of molecules with a high degree of specificity . The binding of the antibodies can then be detected using different techniques. This antigen-antibody reaction forms the basis for numerous experimental and diagnostic procedures (e.g. immunophenotyping , FACS , immunohistology , ELISA , ELISPOT , radioimmunoassay and Western blot ).
Many of the human cell surface antigens recognized by monoclonal antibodies are classified in the CD nomenclature .
Production of monoclonal antibodies
The principle of the production of monoclonal antibodies was published in 1975 by César Milstein , Georges Köhler and Niels Jerne , who received the Nobel Prize in Medicine for it in 1984 . The technique is based on the fusion of antibody-producing B cells with cells of a myeloma - cell line , whereby hybrid cells are formed, the unlimited antibodies of a particular specificity produce ( hybridoma technique ).
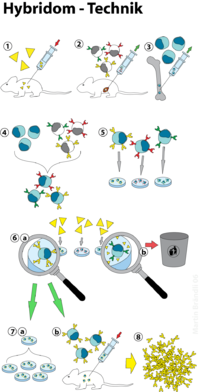
When producing monoclonal antibodies against a specific antigen , a mouse is first infected with this antigen (1, see figure). The immune response leads to the formation of B lymphocytes , which form antibodies which react with the antigen and which accumulate in the spleen . The B lymphocytes are isolated from the removed spleen (2) and fused with cells ( plasma cells ) of a cell line (3) obtained from a myeloma ( plasmacytoma ) (4), so-called hybridoma cell lines (5) are formed. These hybridoma cells combine properties of their original cells: the B lymphocyte has the ability to produce a specific antibody, the myeloma cell the ability to grow indefinitely in vitro (“in a test tube”). The hybridoma cell line that best binds the desired epitope on the antigen is selected to obtain the monoclonal antibody (6). The immortal cell line is preserved and the cell supernatant is harvested regularly as needed. The monoclonal antibodies can be produced in vitro (7a) or in vivo (7b). The antibodies (8) are called monoclonal because they come from a single B-cell of origin and are therefore all identical.
The hybrid cells are selected using a so-called HAT medium. This nutrient medium contains hypoxanthine (a naturally occurring purine derivative), thymidine and aminopterin ( cell poison that inhibits the biosynthesis of purine and pyrimidine bases ). The B lymphocytes and thus also the hybrid cells can metabolize hypoxanthine and thymidine and thus bypass the blockage caused by the aminopterin. The myeloma cells used are deficiency mutants with regard to the alternative metabolic pathway and die in the HAT medium.
The non-fused B-lymphocytes only have a limited lifespan, so that after a few passages only the hybrid cells can be found in the culture.
The technology of phage display represents a major step forward, particularly in the cloning of human antibodies .
Therapeutic Monoclonal Antibodies
Attempts to use monoclonal antibodies in therapy were initially not very successful. The mouse antibodies used (murine antibodies, ending: -omab) act as antigens in the human organism and can trigger an immune response directed against them. The interaction with cells of the recipient's immune system, which is important for their desired effect, was also not optimal due to the different species.
Significant advances have only been made after the development of modified monoclonal antibodies better adapted to human antibodies in recent years.
In addition, antibody conjugates , such as immunocytokines , are also used for therapeutic and diagnostic applications, especially in cancer immunotherapy .
Monoclonal Antibody Terminology
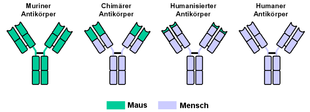
The Free Trade names of all therapeutic monoclonal antibodies carry the suffix "... mab " what for " m onoclonal a nti b is ody". According to the similarity to human antibodies, a distinction is made (in ascending order):
- murine antibodies (from the mouse): ending - omab
- Antibodies from primate: ending - imab
- chimeric antibodies: ending - ximab (only the variable part of the AK is mouse protein.)
- humanized antibodies: ending - zumab (only the antigen binding sites are mouse protein)
- fully human, recombinant antibodies: ending - umab
Lists of antibodies developed
Therapeutic monoclonal antibodies approved or in clinical trials (phase III)
| Surname | Trade name | Type | Target structure | field of use |
|---|---|---|---|---|
| Hematology , oncology | ||||
| Alemtuzumab 5 | MabCampath | humanized | CD52 antigen on lymphocytes | Chronic lymphocytic leukemia , T-cell lymphoma 2 , acute lymphocytic leukemia 2 |
| Apolizumab 1.2 | Remitogenic | humanized | HLA-DR antigen on B lymphocytes | Solid tumors, acute lymphocytic leukemia , chronic lymphocytic leukemia , non-Hodgkin lymphomas |
|
Atezolizumab (MPDL3280A) 1,2 |
Tecentriq ( Roche ) | humanized | PD-L1 | Bladder cancer (approved in USA) CHMP recommendation positive in EU . |
|
Avelumab (MSB0010718C) 2 |
Bavencio ( Merck / Pfizer ) | humane | PD-L1 | Bladder cancer , non-small cell lung cancer (NSCLC), Merkel cell carcinoma |
| Bevacizumab | Avastin ( Roche ) | humanized | VEGF (Vascular Endothelial Growth Factor) | Colon cancer , breast cancer , non-small cell lung cancer , wet, age-related macular degeneration ( off-label use ) 2 |
| Blinatumomab | Blincyto ( Amgen ) | murine, bispecific | CD-19 | ALLES |
| Catumaxomab | Removab ( Neovii Biotech ) | murine (rat / mouse), trifunctional | EpCAM antigen on tumor cells, CD3 receptor on T lymphocytes | malignant ascites due to EpCAM -positive carcinoma |
|
Cemiplimab 1.2 (REGN2810) |
NN, ( Sanofi / Regeneron Pharmaceuticals ) | from primate | PD-1 | Squamous cell carcinoma |
| Cetuximab | Erbitux ( BMS / Merck ) | chimeric | EGF receptor (Epidermal Growth Factor Receptor) | Colon cancer , head and neck tumors |
| Daratumumab 1.2 | Darzalex ( Genmab / Janssen Biotech) | humane | CD38 | Multiple myeloma , already approved in the US and EU |
| Durvalumab 1.2 | Imfinzi ( AstraZeneca / Medimmune ) | humane | PD-L1 | Lung cancer 1,2 |
| Eculizumab | Soliris ( Alexion ) | humanized | C5 complement factor | Paroxysmal nocturnal hemoglobinuria (PNH) |
| Elotuzumab 1.2 | Empliciti ( BMS ) | humanized | Signaling Lymphocyte Activation Molecule (SLAMF7; CS1) | Multiple myeloma , already approved in the USA |
|
Emicizumab 1.2 (ACE910) |
Hemlibra ( Roche ) | humanized, bispecific | Factors IXa and X | Inhibitor hemophilia |
| Epratuzumab 1.2 | LymphoCide ( UCB ) | humanized | CD22 antigen | Non-Hodgkin lymphomas , autoimmune diseases , acute lymphoblastic leukemia |
| Gemtuzumab ozogamicin 1 | Mylotarg ( Wyeth ) | humanized, calicheamicin loaded | CD33 antigen | Acute myeloid leukemia |
| Ibritumomab-Tiuxetan | Zevalin ( Bayer ) | murine, 90 Y -labeled | CD20 antigen on B lymphocytes | Non-Hodgkin lymphoma ( radioimmunotherapy ) |
| Inotuzumab ozogamicin (as conjugate ) 1,2 | Besponsa ( Pfizer ) | humanized | CD22 antigen | Acute lymphoblastic leukemia |
| Ipilimumab | Yervoy ( BMS ) | humane | CTLA-4 | Malignant melanoma |
| Mogamulizumab | Poteligeo ( Kyowa Hakko Kirin ) | humanized | CCR4 | Adult T-cell leukemia , various non-Hodgkin lymphomas |
| Moxetumomab pasudotox-tdfk 1,2 | Lumoxiti ( AstraZeneca ) | murine | CD22 antigen | Hairy cell leukemia |
| Necitumumab 1.2 | Portrazza ( Eli Lilly ) |
humane | EGF receptor (Epidermal Growth Factor Receptor) | Lung cancer , gastric cancer , already approved in NSCLC in the US |
| Nivolumab | BMS) | humane | PD-1 | Approved in Europe for malignant melanoma and non-small cell lung cancer |
| Obinutuzumab | Gazyva | humanized | CD20 antigen on B lymphocytes | Chronic lymphocytic leukemia , follicular lymphoma |
| Ofatumumab | Arzerra ( GSK ) | humane | CD20 antigen on B lymphocytes | Chronic lymphocytic leukemia |
| Olaratum from 1.2 | Lartruvo ( Eli Lilly ) |
humane | IgG1 on PDGF Receptor-α (platelet-derived growth factor receptor α) | Sarcoma ; Revoke admission |
| Oregovomab 1.2 | OvaRex ( United Therapeutics ) | murine | CA-125 | Ovarian cancer |
| Panitumumab | Vectibix ( Amgen ) | humane | EGF receptor (Epidermal Growth Factor Receptor) | EGF receptor expressing tumors, especially metastatic colorectal carcinoma |
| Pembrolizumab (MK-3475) | Keytruda ( MSD ) | humanized | PD-1 | Melanoma , mesothelioma , NSCLC |
| Pertuzumab | Perjeta ( Roche ) | humanized | HER2 / neu , HER2 / neu receptor ; Pertuzumab inhibits the dimerization of the target structure and its heterodimerization with other HER receptors (e.g. EGF receptor (EGFR)), which is said to slow down tumor growth | Breast cancer , clinical studies, etc. a. in ovarian carcinoma , bronchial carcinoma and prostate carcinoma |
| Ramucirumab | Cyramza ( Eli Lilly ) | humane | VEGF receptor 2 | Bronchial carcinoma , gastric carcinoma |
| Rituximab | MabThera ( Roche / Biogen ) | chimeric | CD20 antigen on B lymphocytes | Non-Hodgkin lymphoma |
| Rovalpituzumab tesirine 1,2 | NN ( Stemcentrx ) | humanized | delta-like protein 3 (DLL3) | Small cell lung cancer (SCLC) |
| Siltuximab | Sylvant ( Janssen ) | chimeric | binds human IL-6 | Castleman's disease (MCD), multiple myeloma |
| Tremelimumab 1.2 | NN ( AstraZeneca / Medimmune ) | humane | CTLA-4 | Bronchial carcinoma 1,2 mesothelioma 1,2 |
| Tositumomab 1 | Bexxar ( GSK ) | murine, 131 I -labeled | CD20 antigen on B lymphocytes | Non-Hodgkin lymphoma ( radioimmunotherapy ) |
| Trastuzumab | Herceptin ( Roche ) | humanized | HER2 / neu receptor | Breast cancer , gastric cancer |
| Zanolimumab 1.2 | HuMax-CD4 ( Genmab ) | humane | CD4 antigen on T lymphocytes | T-cell lymphomas |
| Autoimmune diseases , transplant rejection , pain | ||||
| Adalimumab | Humira | humane | TNF-α (Tumor Necrosis Factor α) | Rheumatoid arthritis , psoriatic arthritis , ankylosing spondylitis , Crohn's disease |
| Alemtuzumab 5 | Lemtrada | humanized | CD52 antigen on lymphocytes | multiple sclerosis |
|
Anifrolumab 1.2 (MEDI-546) |
NN | humane | anti-type-I IFN | Lupus erythematosus |
| Basiliximab | Simulect | chimeric | CD25 antigen ( interleukin-2 receptor) | Prophylaxis of acute rejection reactions in kidney transplantation |
| Belimumab | Benlysta | humane | BLys (B-lymphocyte stimulator, a cytokine of the TNF superfamily) | Lupus erythematosus |
| Brodalumab 1.2 | Kyntheum ( LEO Pharma ) | humane | Interleukin-17 receptor | Psoriasis , psoriatic arthritis , asthma |
| Canakinumab | Ilaris | humane | Interleukin-1 beta receptor | Periodic fever syndromes (CAPS, TRAPS, HIDS / MKD, FMF), systemic juvenile idiopathic arthritis (SJIA) , gouty arthritis |
| Certolizumab | Cimzia | humanized | TNF-α (Tumor Necrosis Factor α) | Rheumatoid arthritis , axial spondolyarthritis , psoriatic arthritis , psoriasis , Crohn's disease (not in Germany) |
| Clazakizumab 1.2 | NN ( BMS ) | humanized | Interleukin-6 receptor | Rheumatoid arthritis |
| Daclizumab | Zenapax (aH); Zinbryta (aH) | humanized | CD25 antigen ( interleukin-2 receptor) | Multiple sclerosis , but taken off the market (aH) |
| Epratuzumab 1.2 | LymphoCide | humanized | CD22 antigen | Autoimmune diseases , non-Hodgkin lymphomas , |
| Fasinum from 1.2 | NN ( Regeneron Pharmaceuticals ) | humane | Nerve Growth Factor (NGF) | Osteoarthritis pain |
| Guselkumab 1.2 | Tremfya
( Janssen ) |
humane | Anti-interleukin-23 | Psoriasis , rheumatoid arthritis |
| Golimumab | Simponi | humane | TNF-α (Tumor Necrosis Factor α) | Ulcerative colitis , rheumatoid arthritis , psoriatic arthritis , ankylosing spondylitis |
| Infliximab | Remicade | chimeric | TNF-α (Tumor Necrosis Factor α) | Crohn's disease , rheumatoid arthritis , ankylosing spondylitis , psoriatic arthritis , ulcerative colitis |
| Ixekizumab | Taltz | humanized | Interleukin-17 A receptor (IL-17A) | Psoriasis , psoriatic arthritis |
| Mavrilimumab (CAM-3001) 1.2 | NN ( AstraZeneca ) | humane | Rheumatoid arthritis | |
| Muromonab CD3 | Orthoclone OKT3 | murine | CD3 receptor on T lymphocytes | Treatment of acute rejection reactions in kidney, heart and liver transplants |
| Natalizumab 3 | Tysabri | humanized | CD49d (α 4 integrin) | multiple sclerosis |
| Risankizumab 1.2 | Skyrizi ( AbbVie ) | humanized | Interleukin-23 receptor | Psoriasis , psoriatic arthritis , Crohn's disease |
| Rituximab | MabThera | chimeric | CD20 antigen on B lymphocytes | Rheumatoid arthritis |
| Sarilumab | Kevzara | humane | Interleukin-6 receptor (IL-6R) | Rheumatoid arthritis |
| Secukinumab | Cosentyx ( Novartis ) | humane | Interleukin-17 A receptor | Psoriasis , psoriatic arthritis , ankylosing spondylitis |
| Sifalimumab (MEDI-545) 1.2 | NN ( AstraZeneca ) | humane | Lupus erythematosus | |
| Sirukumab 1.2 | Plivensia ( Janssen Pharmaceutica / GlaxoSmithKline ) | humane | Interleukin-6 receptor | Rheumatoid arthritis , but negative recommendation from the FDA |
| Tocilizumab | RoActemra | humanized | Interleukin-6 receptor | Rheumatoid arthritis , cytokine storm after treatment with CART cells, giant cell arteritis |
| Ustekinumab | Stelara | humane | Interleukin 12/23 | Plaque psoriasis |
| Vedolizumab 1.2 | Entyvio | humanized | IgG1 | Crohn's disease and ulcerative colitis |
| Hypercholesterolemia | ||||
| Alirocumab | Praluent ( Sanofi ) | humane | PCSK9 (indirect: LDL receptor ) | Hypercholesterolemia (prevention of cardiovascular events including stroke ) |
|
Evinacumab (REGN1500) 1.2 |
NN (Regeneron / Sanofi) | humane | PCSK9 | Homozygous familial hypercholesterolemia |
|
Evolocumab (AMG-145) |
Repatha ( Amgen ) | humane | PCSK9 (indirect: LDL receptor ) | Hypercholesterolemia (prevention of cardiovascular events including stroke ) |
| Cardiovascular diseases | ||||
| Abciximab | ReoPro | chimeric, Fab fragment | GPIIb / IIIa on platelets | Prevention of vascular occlusion after PTCA , discontinued |
| Special forms of therapy | ||||
| Idarucizumab | Praxbind | humanized | Antibody fragment (Fab) that binds to dabigatran with a very high affinity . | Antidote for the rapid and specific abolition of the anticoagulant caused by dabigatran |
| Infectious diseases | ||||
| Bezlotoxumab 1.2 | Zinplava ( MSD ) | humane | Clostridium difficile toxin B. | Clostridium difficile infection (CDI) |
| Motavizumab 4 | Numax | chimeric | Part of the respiratory syncytial virus (RSV) | Prophylaxis of RSV (respiratory syncytial virus) pneumonia in premature babies , ELBW (Extremely Low Birth Weight Preterm) and VLBW (Very Low Birth Weight Preterm) |
| Palivizumab | Synagis | humanized | Part of the respiratory syncytial virus (RSV) | Prophylaxis of RSV pneumonia in premature infants |
| Ibalizumab-uiyk | Trogarzo | humanized | CD4 T cell receptor | AIDS |
| Neurological diseases | ||||
| Aducanumab 1.2 | NN ( Biogen ) |
humane | tbd. | Alzheimer |
| Eptinezumab | NN | humanized | Calcitonin Gene-Related Peptide (CGRP) | migraine |
| Erenumab | Aimovig ( Amgen ) |
humane | Calcitonin Gene-Related Peptide (CGRP) receptor | migraine |
| Fremanezumab 1.2 | NN ( Teva ) |
humanized | Calcitonin Gene-Related Peptide (CGRP) |
Migraine cluster headache |
| Galcanezumab 1.2 | Emgality ( Eli Lilly ) |
humanized | Calcitonin Gene-Related Peptide (CGRP) |
Migraine cluster headache |
| Ocrelizumab 1.2 | Ocrevus ( Roche ) |
humanized | CD20 antigen | multiple sclerosis |
| Solanezumab 1.2 | NN ( Eli Lilly ) |
humanized | tbd. | Alzheimer's disease |
| Ublituximab | NN ( TG Therapeutics ) | chimeric (mouse / human) | CD20 antigen on B lymphocytes | multiple sclerosis |
| Ophthalmology | ||||
| Ranibizumab | Lucentis | humanized, Fab fragment | VEGF-A (Vascular Endothelial Growth Factor A) | Wet macular degeneration |
| dermatology | ||||
| Adalimumab | Humira | humane | TNF-α (Tumor Necrosis Factor α) | psoriasis |
| Dupilumab | Dupixent ( Sanofi ) | humane | Interleukin 4/13 | Atopic eczema sinusitis |
| Efalizumab | Raptiva | humanized | CD11a antigen | psoriasis |
| Infliximab | Remicade | chimeric | TNF-α (Tumor Necrosis Factor α) | psoriasis |
| Nemolizumab 1.2 | NN ( Chugai ) | humanized | Interleukin 31 | Atopic eczema |
| Tildrakizumab | Ilumetri | humane | Interleukin 23 | Moderate to severe plaque psoriasis |
| Ustekinumab | Stelara | humane | Interleukin 12/23 | Plaque psoriasis |
| Allergic diseases | ||||
| Benralizumab | Fasenra (AstraZeneca) | humanized | Alpha subunit of the IL-5 receptor (IL-5Rα) | Asthma ; So far tested in COPD , but not successful |
| Mepolizumab | Nucala ( GSK ) | humanized | Interleukin-5 | Asthma , COPD , hypereosinophilia syndrome , eosinophilic granulomatosis with polyangiitis |
| Omalizumab | Xolair | humanized | IgE (F c part) | Severe bronchial asthma , chronic idiopathic urticaria ( hives ) |
| Reslizumab | Cinqaero ( Teva ) | humanized | Interleukin-5 | asthma |
| Tralokinumab 1.2 | NN ( AstraZeneca / Medimmune) | humanized | IL-13 | Severe bronchial asthma, atopic eczema |
| Dentistry | ||||
| ( plantibody ) 1,2 | CaroRx | produced recombinantly in plants ("plantibody") | specific binding to Streptococcus mutans (key germ of dental caries) | as a mouth rinse against dental caries ; Elimination of S. mutans from the oral flora |
| Osteology | ||||
| Denosumab | Prolia, XGEVA | humane | RANK ligand (receptor activator of the NF-κB ligand, RANKL ) | Osteoporosis ; Bone metastases |
| Romosozumab | Evenity ( Amgen / UCB ) | humanized | Sclerostin | postmenopausal osteoporosis |
1 Not yet approved in Germany or in approval (may no longer be up-to-date)
2 In clinical studies
3 Approved again in the USA by the FDA under strict conditions despite possible rare serious side effects , European approval since 6/2006
4 Development discontinued.
5 Removed from the market by the manufacturer - as MabCampath - in order to bring the substance back onto the market under a new trade name and a different indication (MS). Criticized by the drug commission of the German medical profession (AkdÄ).
For in vivo diagnostic monoclonal antibody approved
| Surname | preparation | Type | Target structure | field of use |
|---|---|---|---|---|
| Sulesomab | LeukoScan ® | murine, 99m Tc -labeled | IMMU-MN3 Fab'-SH fragment against granulocytes | Osteomyelitis |
| Arcitumomab | CEA-Scan ® | murine, 99m Tc -labeled | IMMU-4 F (ab ') 2 versus CEA | colorectal cancer |
Withdrawn or discontinued diagnostic monoclonal antibodies
| Surname | preparation | Type | Target structure | Planned areas of application | Complications and comment |
|---|---|---|---|---|---|
| Igovomab | Indimacis 125 | murine, 111 labeled In | CA 125 antigen | ovarian serous adenocarcinomas | Removed from the European market in 1999 at the request of the manufacturer. Reason? |
Therapeutic monoclonal antibodies in preclinical or Phase I / II trials
| Surname | preparation | Type | Target structure | field of use |
|---|---|---|---|---|
| Atrosab | - | humanized | CD120a (TNFR1) | Crohn's disease , multiple sclerosis , rheumatoid arthritis |
| Brentuximab | - | chimeric | CD30 | Lymphoma |
| Cantuzumab | - | humanized, mersantine conjugated | CanAg ( MUC1 ), antibody conjugated with mersantin (toxin) | Colon cancer , gastric cancer , pancreatic cancer , NSCLC |
| Labetuzumab | - | humanized | Carcinoembryonic Antigen (CEA) | Colon cancer , pancreatic cancer , ovarian cancer |
| Nimotuzumab | TheraCim ® | humanized | EGF receptor (Epidermal Growth Factor Receptor) | metastatic irinotecan refractory colorectal cancer |
| Mapatumumab | - | humane | - | Colon cancer |
| Matuzumab | EMD72000 | humanized | EGF receptor (Epidermal Growth Factor Receptor) | Gastric cancer , colon cancer , NSCLC |
| Pertuzumab | Omnitarg ® | humanized | HER2 / new | Breast cancer , prostate cancer , ovarian cancer , NSCLC |
| R1450 | - | humane | Amyloid-β | Alzheimer's disease |
| 1D09C3 | - | humane | MHC-II | Non-Hodgkin lymphoma (NHL) |
Withdrawn or discontinued therapeutic monoclonal antibodies
| Surname | preparation | Type | Target structure | Planned areas of application | Complications and comment |
|---|---|---|---|---|---|
| Bococizumab | NN ( Pfizer ) | humanized | PCSK9 (indirect: LDL receptor ) | Hypercholesterolemia | Pfizer has stopped further development. |
| Briakinumab | - | p40 subunit of interleukin-12 (IL-12) and interleukin-23 (IL-23) | psoriasis | Abbott's application for approval withdrawn | |
| Galiximab | - | chimeric | CD80 antigen | Non-Hodgkin lymphoma | |
| Lumiliximab | - | chimeric (macaque / human) | CD23 antigen on B lymphocytes | Chronic lymphocytic leukemia | |
| Nebacumab | Centoxin ® | humanized (IgM) | Endotoxin | sepsis | Approval in Europe in 1991, withdrawn from the market in 1993 due to increased patient mortality after treatment with nebacumab compared to placebo. |
| Edrecolomab | Panorex ® | Mouse IgG2a | EpCAM | Colon cancer | Approval in Germany in 1995, withdrawn from the market in 2000 because the previous standard therapy was more effective. |
| TGN1412 | - | humanized | CD28 | Leukemia and autoimmune diseases (such as multiple sclerosis and rheumatism ) | Cytokine storm . In the public criticism were shortcomings in the test planning and execution, z. B. that the preparation was given to 6 subjects at the same time, and that the mechanisms of action were not understood. |
Individual evidence
- ^ G. Koehler, C. Milstein: Continuous cultures of fused cells secreting antibody of predefined specificity. In: Nature. Vol. 256, 1975, pp. 495-497. PMID 1172191 . doi: 10.1038 / 256495a0 , reprinted in: J. Immunol. Vol. 174, pp. 2453-2455. PMID 15728446 , jimmunol.org (PDF)
- ↑ Information from the Nobel Foundation on the 1984 award ceremony to César Milstein, Georges Köhler and Niels Jerne (English)
- ↑ Hans Bojar: How a cancer immunotherapy works. Hans Bojar, accessed November 27, 2019 .
- ↑ Tess Stynes: FDA Approves Roche Immunotherapy for Bladder Cancer. In: wsj.com. May 18, 2016, accessed May 30, 2016 .
- ↑ CHMP recommends EU approval for Roche's TECENTRIQ (atezolizumab) in a specific type of metastatic lung and two types of metastatic bladder cancer , PM Roche of July 21, 2017, accessed on August 10, 2017
- ↑ Clinical study (phase 3): Avelumab in Non-Small Cell Lung Cancer (JAVELIN Lung 200) at Clinicaltrials.gov of the NIH
- ↑ Positive opinion of the CHMP of the EMA for avelumab in metastatic Merkel cell carcinoma ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Merck PM on July 21, 2017, accessed July 28, 2017
- ↑ Sanofi and Regeneron Announce That Cemiplimab (REGN2810) Has Received FDA Breakthrough Therapy Designation for Advanced Cutaneous Squamous Cell Carcinoma , PM SanofiGenzyme, September 8, 2017, accessed September 10, 2017
- ↑ FDA approves Darzalex for patients with previously treated multiple myeloma , PM FDA dated November 16, 2015, accessed November 20, 2015
- ↑ Janssen's single agent DARZALEX® (daratumumab) Approved by the European Commission for Treatment of Multiple Myeloma (MM) | Business Wire. In: www.businesswire.com. Retrieved May 23, 2016 .
- ↑ FDA approves Empliciti, a new immune-stimulating therapy to treat multiple myeloma , PM FDA dated November 30, 2015, accessed December 1, 2015
- ↑ Roche's emicizumab for haemophilia A meets primary endpoint in phase III study , PM Roche of December 22, 2016, accessed on January 5, 2017
- ↑ US FDA approves Lumoxiti (moxetumomab pasudotox-tdfk) for certain patients with relapsed or refractory hairy cell leukaemia , PM AstraZeneca of September 14, 2018, accessed on September 14, 2018
- ↑ FDA approves Portrazza to treat advanced squamous non-small cell lung cancer , PM FDA of November 24, 2015, accessed November 25, 2015
- ↑ PZ: Pharmazeutische Zeitung online: Nivolumab now also against lung cancer. In: pharmische-zeitung.de. July 21, 2015, accessed May 16, 2016 .
- ↑ Pharm. Ztg. Online, drug profile : Olaratumab (accessed on August 3, 2019)
- ↑ Biogen and AbbVie announce the independent worldwide withdrawal of the marketing authorization of Zinbryta (daclizumab) for the treatment of multiple sclerosis , PM Biogen of March 2, 2018, accessed on March 15, 2018
- ↑ Positive Opinion , PM EMA February 28, 2019, accessed March 15, 2019
- ↑ FDA ADVISORY COMMITTEE DOES NOT RECOMMEND APPROVAL OF SIRUKUMAB FOR THE TREATMENT OF MODERATELY TO SEVERELY ACTIVE RHEUMATOID ARTHRITIS , PM JNJ of August 2, 2017, accessed August 14, 2017
- ↑ Roche gains positive CHMP opinion for Actemra / RoActemra in giant cell arteritis , Roche PM July 21, 2017, accessed August 10, 2017
- ↑ Jannsen-Cilag GmbH: Information letter: Voluntary discontinuation of sales of ReoPro® (Abciximab) in Germany on December 15, 2018. December 12, 2018, accessed September 19, 2019 .
- ↑ SUMMARY OF MEDICINAL PRODUCT CHARACTERISTICS , EMA, accessed May 23, 2017
- ↑ FDA Approves Merck's ZINPLAVA ™ (bezlotoxumab) to Reduce Recurrence of Clostridium difficile Infection (CDI) in Adult Patients Receiving Antibacterial Drug Treatment for CDI Who Are at High Risk of CDI Recurrence , Merck PM, October 21, 2016, accessed January 5 2017
- ↑ AstraZeneca discontinues development of motavizumab for RSV prophylaxis indication . AstraZeneca terminates development of motavizumab in RSV, AstraZeneca press release dated December 21, 2010
- ↑ Motavizumab in the English language Wikipedia
- ↑ Doctors newspaper: International Headache Congress: New antibody therapies against migraines in sight. Retrieved March 9, 2018 .
- ↑ David W Dodick, Peter J Goadsby, Stephen D Silberstein, Richard B Lipton, Jes Olesen, Messoud Ashina, Kerri Wilks, David Kudrow, Robin Kroll, Bruce Kohrman, Robert Bargar, Joe Hirman, Jeff Smith: Safety and efficacy of eptinizumab, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomized, double-blind, placebo-controlled, exploratory phase 2 trial . In: The Lancet Neurology . 13, No. 11, 2014, p. 1100. doi : 10.1016 / S1474-4422 (14) 70209-1 . PMID 25297013 .
- ↑ FDA Accepts Biologics License Application For Aimovig ™ (erenumab) , Amgen PM, July 20, 2017, accessed July 26, 2017
- ↑ FDA Approves Aimovig ™ (erenumab-aooe), A Novel Treatment Developed Specifically For Migraine Prevention , PM Amgen, May 17, 2018, accessed May 19, 2018
- ↑ Aimovig ( Memento of the original from May 18, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Multimedia News Release, accessed May 19, 2018
- ↑ Doctors newspaper: International Headache Congress: New antibody therapies against migraines in sight. Retrieved March 9, 2018 .
- ↑ A Study to Evaluate the Efficacy and Safety of TEV-48125 (Fremanezumab) for the Prevention of Episodic Cluster Headache (ECH). ClinicalTrials.gov Identifier: NCT02945046.
- ↑ Doctors newspaper: International Headache Congress: New antibody therapies against migraines in sight. Retrieved March 9, 2018 .
- ↑ Emgality - Opinion , EMA of September 20, 2018, accessed on September 27, 2018
- ↑ A Study of LY2951742 in Participants With Episodic cluster headache. ClinicalTrials.gov Identifier: NCT02397473
- ↑ Article in The New England Journal of Medicine dated October 1, 2016 Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis
- ↑ Philipp Kressirer: New active ingredient against neurodermatitis. In: IDW Informationsdienst Wissenschaft. idw-online.de, October 5, 2016, accessed on October 11, 2016 .
- ↑ rme / aerzteblatt.de: Atopic dermatitis: Dupilumab shines in phase 3 studies. In: Deutsches Ärzteblatt . Source: Clinical Trials (SOLO 1 and SOLO 2 studies), October 4, 2016, accessed October 17, 2016 .
- ↑ Summary of the EPAR for the public , EMA, accessed on October 27, 2017
- ↑ GALATHEA Phase III trial did not meet the primary endpoint of a statistically-significant reduction of exacerbations in patients with COPD , PM AstraZeneca of May 11, 2018, accessed on May 20, 2018
- ↑ CaroRx . planetbiotechnology.com
- ↑ Withdrawal of MabCampath ® (PDF; 114 kB) Information letter from Genzyme, a company of the Sanofi Group, dated August 10, 2012.
- ↑ Information and statement from the AkdÄ on the withdrawal of MabCampath ® (alemtuzumab) from the AkdÄ newsletter of August 24, 2012.
- ^ Community list of not active medicinal products for human use, accessed on August 5, 2007 .
- ↑ Clinical Trials: R1450 . Roche
- ↑ Monoclonal cancer antibody 1D09C3 receives orphan drug status for chronic lymphocytic leukemia from the European Commission ( Memento of the original from February 22, 2014 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ^ Pfizer Discontinues Global Development of Bococizumab, Its Investigational PCSK9 Inhibitor , Pfizer PM November 1, 2016, accessed January 5, 2017
- ↑ Article on nebacumab. In: New York Times