Aminopterin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
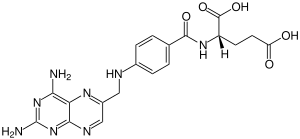
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname |
|
|||||||||||||||||||||
| other names |
4'-deoxy-4'-aminofolic acid |
|||||||||||||||||||||
| Molecular formula | C 19 H 20 N 8 O 5 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 440.42 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
225 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
heavy in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aminopterin is an analogue of folic acid (vitamin B 9 ) and acts biologically as a folic acid inhibitor. Chemically it is a synthetic pterin - derivative with antineoplastic (against cancer -directed) and immunosuppressive properties.
Aminopterin was previously used as a cytostatic agent, but is now largely replaced by the structurally similar methotrexate . In cell culture , aminopterin is often used in selection media, particularly in the production of monoclonal antibodies .
The connection was significant due to a large recall campaign of dog and cat food in the United States in 2007 , in which around one hundred finished feeds, including those from well-known manufacturers, were withdrawn from the market. Aminopterin contamination was discussed as a possible cause of feed-induced kidney failure .
application
The cytostatic effect of the folic acid inhibitor aminopterin was first used in 1947 by Sidney Farber in children with leukemia , whereby a temporary remission (regression, healing) of the cancer could be achieved. In the years between 1953 and 1964, aminopterin was marketed in the United States by Lederle Laboratories, later taken over by the pharmaceutical company Wyeth , for the indication of ' pediatric leukemia'. At the same time, the company was already selling the structurally similar methotrexate. Due to difficulties in the manufacture of the substance, Lederle Laboratories later discontinued the production of aminopterin in favor of methotrexate.
In addition to its actual use as a cytostatic drug, the drug was used in the United States in the same period in over 4,000 patients with great success for the treatment of psoriasis : the lesions apparently receded without any serious side effects being observed.
In the 1950s, aminopterin was replaced as a cancer drug by methotrexate, as the latter showed a greater therapeutic range in a mouse tumor model .
In the 1960s and before, the substance was also tested as an abortifacient . After this, however, is associated with congenital malformations came, this application was not pursued comparable teratogenic effects were also demonstrated for methotrexate, so that the teratogenic effects of folic acid antagonists, the term (fetal) aminopterin syndrome are summarized. This is a complex syndrome with severe changes in the skull bone and formation of club feet . Affected children are rarely born alive and if they do, they usually do not survive the first year of life. Such severe developmental disorders of the early fetus ( embryopathies ) can occur if folic acid inhibitors are taken in the 4th to 12th week of pregnancy, the risk of damage to the embryo for aminopterin is estimated at 85% and for methotrexate at 50% .
Although z. For example, it is often claimed on the Internet that aminopterin is also used as a rodenticide (e.g. rat poison ), there is no evidence that it was ever actually used for such a purpose. On the one hand, this is contradicted by the fact that the production of the substance is complex and expensive. On the other hand, aminopterin is an environmentally unstable molecule that breaks down when exposed to light and heat . The erroneous assumption that aminopterin is a rodent control agent goes back to a patent granted to the American Cyanamid Company (the parent company of Lederle Laboratories) in 1951 and which is widely cited in various specialist books and other sources .
Today aminopterin is mainly used in the production of monoclonal antibodies for the selection of hybridoma cells as part of the hybridoma technology founded in 1975 by César Milstein and Georges Köhler .
pharmacology
As a folate analog aminopterin blocked by competitive inhibition of the dihydrofolate reductase , an enzyme which dihydrofolic reduced . By blocking the synthesis of tetrahydrofolic acid - it functions as an important methyl group donor in the metabolism of all living things - there is a lack of nucleotide precursors , which prevents the synthesis of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), which is necessary for cell division . This leads to a breakdown in protein synthesis and thus to the decline of the affected cells and tissues.
In addition, aminopterin is toxic, which is why it is no longer used in medicine. The lethal dose (LD) for mice administered orally as a single dose is 3 mg / kg.
Feed scandal
Aminopterin was detected in 2007 by the New York State Food Laboratory at levels of at least 40 ppm in approximately one hundred ready-to-eat dog and cat foods . The laboratory had been commissioned to analyze the samples, as severe kidney diseases were suddenly observed in dogs and cats as part of a feed scandal from February 2007. Since higher doses of methotrexate crystallize in the renal tubules and cause kidney damage, a similar mechanism was assumed for aminopterin. The direct connection between aminopterin and the feed-related diseases has not been proven and is considered unlikely. Instead, melamine and cyanuric acid , which had caused the disease by the formation of insoluble crystals in the renal tubules , were found in the same feed samples .
It is not possible to detect aminopterin in living animals. Antioxidants and folinic acid can be used as a therapeutic attempt, but their effect as an antidote after long-term exposure to aminopterin has not been proven.
Web links
- AVMA on the recall campaign ( Memento from November 27, 2008 in the Internet Archive )
Individual evidence
- ↑ a b c d Entry on N- (4 - [[(2,4-diamino-6-pteridinyl) methyl] amino] benzoyl) -L-glutamic acid in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on aminopterin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Who was Sidney Farber, MD? . Dana-Farber Cancer Institute . Retrieved February 5, 2007.
- ↑ Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA: Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin) . In: N Engl J Med . 238, No. 787, 1948, pp. 787-93. PMID 18860765
- ↑ Rees, RB, JH Bennett et al: Aminopterin for psoriasis: A decade's observation. In: Archives of Dermatology Volume 90, page 544, 1964. PMID 14206858
- ↑ A. GOLDIN, JM VENDITTI, SR HUMPHREYS, D. DENNIS, N. MANTEL, SW GREENHOUSE: A quantitative comparison of the antileukemic effectiveness of two folic acid antagonists in mice. In: Journal of the National Cancer Institute. Volume 15, Number 6, June 1955, pp. 1657-1664, PMID 14381889 .
- ^ DJ EMERSON: Congenital malformation due to attempted abortion with aminopterin. In: American Journal of Obstetrics and Gynecology . Volume 84, August 1962, pp. 356-357, PMID 13890101 .
- ↑ Leonhard Doederlein: foot deformities 1. The clubfoot. Springer, 1999, ISBN 978-3-540-65622-7 ( limited preview in Google book search).
- ↑ No Aminopterin in Tissues of Animals Killed by Recalled Pet Food . PRNewsWire. March 30, 2007. Retrieved April 14, 2007.
- ^ Alfred L. Franklin. United States Patent Number 2,575,168. Rodenticide comprising 4-amino-pteroylglutamic acid. American Cyanamid Company, New York, NY. November 13, 1951.
- ↑ toilet Werkheiser: THE BIOCHEMICAL, CELLULAR, AND Pharmacological ACTION AND EFFECTS OF THE FOLIC ACID antagonists. In: Cancer Res. 1963 Sep; 23: 1277-85. ( Full text ; PDF; 1.6 MB).
- ↑ M. Johnson: NY Lab Conducting More Pet Food Tests. In: Washington Post, March 27, 2007, accessed December 9, 2014.

