Cyanuric acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
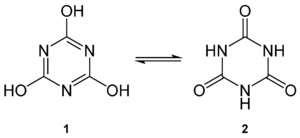
| Cyanuric acid ( 1 ) and the tautomeric form isocyanuric acid ( 2 ) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyanuric acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 3 N 3 O 3 | |||||||||||||||
| Brief description |
white, crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 129.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.75 g cm −3 (25 ° C, anhydrous) |
|||||||||||||||
| Melting point |
320 ° C (decomposition) |
|||||||||||||||
| solubility |
heavy in water (2 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cyanuric acid (1,3,5- triazine -2,4,6-tri ol ) and isocyanuric acid (1,3,5-triazine-2,4,6-tri on ) each other tautomeric chemical compounds of the empirical formula C 3 H 3 N 3 O 3 . Cyanuric acid forms colorless crystals that contain 2 moles of water of crystallization per mole of cyanuric acid , which escape in dry air . The compound is one of the heterocycles ; only cyanuric acid has an aromatic character.
Extraction and presentation
Cyanuric acid can be obtained by hydrolysis of cyanuric chloride , the decomposition of melamine by acids or by heating biuret or urea to 200–300 ° C. On an industrial scale, cyanuric acid is obtained by pyrolysis of urea. The reaction starts at approx. 175 ° C.
properties
Cyanuric acid and isocyanuric acid are the trimerization product of cyanic acid and are in equilibrium , which means that one form can change into the other. Therefore, cyanuric acid and isocyanuric acid can only be distinguished in their derivatives .
In water the compound is slightly soluble, wherein the pH of the saturated solution is between 3.8 and 4. FIG.
When heated, cyanuric acid can break down into three parts in a violent reaction .
use
In particular, cyanuric acid is used as a starting material for the trichloroisocyanuric acid synthesis. This is an important disinfectant and is also used for chlorinating swimming pools. The problem is the accumulation of cyanuric acid in the water, as this reduces the effectiveness of the chlorine. With higher concentrations of cyanuric acid in the pool water (from around 40 mg / l), the dosage of chlorine-based disinfectants must therefore be increased in order to achieve consistent germicidal action.
However, cyanuric acid is also used in the manufacture of paint auxiliaries and as a starting material for cyanic acid extraction on a laboratory scale.
Isocyanuric acid is used as a raw material for the production of triglycidyl isocyanurate , a hardener for powder coatings .
Web links
- Description of Chemical land 21
Individual evidence
- ↑ a b c d Entry on cyanuric acid in the GESTIS substance database of the IFA , accessed on May 13, 2017(JavaScript required) .
- ↑ a b Cyanuric acid data sheet from Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ Data sheet cyanuric acid (PDF) from Merck , accessed on January 19, 2011.
- ^ Klaus Huthmacher, Dieter Most: Cyanuric Acid and Cyanuric Chloride . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005. doi : 10.1002 / 14356007.a08_191