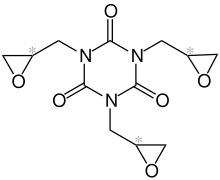
Triglycidyl isocyanurate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Stereoisomene mixture simplified structural formula without stereochemistry |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triglycidyl isocyanurate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 15 N 3 O 6 | |||||||||||||||
| Brief description |
white powder or granules |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 297.27 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.434 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
92.8 ° C |
|||||||||||||||
| solubility |
heavy in water (9 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : mutagenic ( CMR ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triglycidyl isocyanurate (TGIC) is a heterocyclic chemical compound . The crystalline substance has a central six-membered isocyanuric acid and three epoxy substituents. In granulated delivery form, it is usually used as a hardener in weather-resistant powder coatings based on polyester resins.
Extraction
Isocyanuric acid is reacted with an excess of epichlorohydrin in the presence of a tetraalkylammonium salt as a catalyst .
Chemical properties
Due to its three terminal epoxy groups, TGIC can crosslink with branched carboxy groups .
use
Powder coatings crosslinked with TGIC, based on polyester resins containing carboxy groups, are characterized by good weathering stability. The TGIC reacts by means of its epoxy groups with the carboxy groups of the polyester resin via a polyaddition reaction , i.e. without cleavage products. The resulting network makes the resulting powder coating very resistant to aggressive ultraviolet radiation . At the same time, TGIC-crosslinked powder coatings show good flexibility with a comparatively satisfactory chemical resistance.
Due to its mutagenic properties (mutagenic category 2 M: 2), and the classification as toxic since mid-1998 , its use as a hardener for powder coatings has decreased significantly in Europe. Current externally resistant powder coatings are therefore increasingly crosslinked with alternative hardeners, for example based on hydroxylalkylamide .
The alpha isomer of TGIC was used experimentally as an active ingredient against tumors under the names Teroxirone , Alpha-TGT and Henkel's Compound in the 1990s. However, the clinical trials were not continued.
proof
TGIC can be detected by means of liquid chromatography (HPLC), whereby TGIC must first be extracted from powder coatings using tetrahydrofuran . This extraction can be dispensed with in the case of non-bound TGIC.
Safety instructions / risk assessment
The most common way of contact with TGIC for humans is during the manufacture of the substance itself, as well as during the processing of the powder coatings made from it. Most critical here is the inhalation of dusts from pure TGIC. In order to avoid this, the use of respiratory protection and suction is absolutely necessary. Due to the possibility of skin and eye irritation, the use of protective goggles and protective gloves must be observed. When processing powder coatings containing TGIC, a clear bioavailability could not be proven.
Triglycidyl isocyanurate was included by the EU in 2012 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation in the Community's ongoing action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of triglycidyl isocyanurate were concerns about its classification as a CMR substance, consumer use , environmental exposure, exposure of workers and widespread use as well as the dangers arising from a possible assignment to the group of PBT / vPvB substances. The re-evaluation took place from 2015 and was carried out by Poland . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i Entry on triglycidyl isocyanurate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on 1,3,5-tris (oxiranylmethyl) -1,3,5-triazine-2,4,6 (1H, 3H, 5H) -trione in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can extend the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on October 17, 2015.
- ^ J. Pietschmann: Industrial powder coating. 2nd Edition. Vieweg & Sohn Verlag, Wiesbaden 2003, ISBN 3-528-13380-5 .
- ↑ Federal Institute for Occupational Safety and Health, Proof of Genotoxicity of TGIC, Edition: November 1997 .
- ↑ National Industrial Chemicals Notification and Assessment Scheme, Priority Existing Chemical No. 1 , edition: April 1994 ( Memento from May 14, 2013 in the Internet Archive ) (PDF; 716 kB).
- ↑ BVGR online database: Procedure for determining triglycidyl isocyanurate (TGIC) ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Edition: April 1999.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1,3,5-Tris (oxiran-2-ylmethyl) -1,3,5-triazinane-2,4,6-trione (TGIC) , accessed on March 26, 2019.