Cyanic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
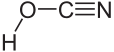
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyanic acid | |||||||||||||||
| Molecular formula | HOCN | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 43.02 g mol −1 | |||||||||||||||
| pK s value |
3.7 (25 ° C) ( isocyanic acid ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The cyanuric acid is an unstable Cyansauerstoffsäure which possibly is obtained in tracks on acidification of cyanates. However, its tautomer isocyanic acid is produced almost exclusively . Cyanic acid could be clearly detected after irradiating isocyanic acid with UV light (224 nm ) in an argon or nitrogen matrix at 4 or 20 K.
Cyanates
Cyanates are the salts with the cyanate ion (NCO - ) or the esters of cyanic acid with the structure R – OCN. Inorganic cyanates such as potassium cyanate are stable, water-soluble compounds and are colorless. Cyanic acid esters generally trimerize to cyanuric acid triesters . In the case of alkyl cyanates, this happens very quickly (even during production), in the case of aryl cyanates, it happens slowly.
use
The free acid is not known to be used because of its instability. Inorganic cyanates are used as auxiliaries in the heat treatment of materials from steel and as starting materials for the production of pharmaceuticals and urea - herbicides used.
See also
Individual evidence
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-42.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ Hans Beyer , Wolfgang Walter : Textbook of organic chemistry . 24th edition. Hirzel, Stuttgart 2004, ISBN 3-7776-1221-9 .
- ↑ Entry on cyanic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
literature
- Entry to cyanic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.