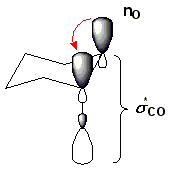Anomeric effect
In organic chemistry , the anomeric effect describes the tendency of atoms in certain molecular structures to preferentially occupy a certain spatial position, since this is energetically more favorable.
Technically precisely formulated, a heteroatom which is substituted on a carbon atom next to a heteroatom in the cyclohexane ring preferably occupies the axial position compared to the (sterically less hindered!) Equatorial position, which contradicts steric considerations. In other words, when two electronegative substituents are present on a carbon atom, the synclinal arrangement of two bonds is preferred over the antiperiplanar arrangement. The carbon atom to which the relevant substituents are attached is referred to as the anomeric carbon . In the formula scheme, the formation of molecule 2 with a synclinal arrangement of the bonds marked in red is preferred to 1 .

This stereoelectronic effect also occurs individually for each of the molecules shown, since they can invert their chair conformation. An axially positioned hydroxyl function is thus converted into an equatorial one and vice versa; Here too, the conformation in which the OH group is axial is preferred in both cases.
The anomeric effect was first observed in pyrans .
There are two approaches to explaining this effect. The simpler explanation is based on classical physics . In the equatorial conformation, the dipoles of both heteroatoms are aligned in one plane, so that the dipole character of the antiperiplanar position is high overall. In the synclinal position, the dipoles of the bonds marked in red in the picture cancel each other out partially, which leads to an energetically lower and therefore more stable conformation. The more complex explanation is based on quantum mechanics and is widely accepted. Negative hyperconjugation takes place between the lone pair of electrons of the hetero atom and the σ * orbital of the CX bond , which is energetically favorable. This is illustrated in the illustration below, the atomic names for the oxygen atoms and the second free electron pair of the rear oxygen atom being hidden for the sake of clarity. It is likely that both electrostatic and quantum mechanical causes cause the anomeric effect.
literature
- Hans Beyer, Wolfgang Walter: Textbook of organic chemistry , S. Hirzel Verlag, Stuttgart, 19th edition, ISBN 3-7776-0356-2 .
- P. Collins, R. Ferrier: Monosacharides - Their Chemistry and their Roles in Natural Products , Wiley West Sussex 1995, ISBN 0-471-95343-1 .