Analogue (chemistry)
Analogs are chemical compounds that have either structural or functional similarity. Accordingly, a distinction is made between structural analogues and functional analogues .
Structural analogs can also produce similar biological effects due to their structural similarity or similar charge distribution , since they are bound by the same receptors and thus trigger similar metabolic reactions or signal cascades . They rarely have identical, mostly similar, but often completely different properties than the original molecule . Particularly with regard to biological systems , such properties can only be found on living objects ( in vivo ) through extensive scientific studies. There are often serious side effects even only after longer periods of time in studies with a large number of study participants.
In contrast to the analogues, there are bioidentical substances, the molecules of which are identical to the naturally occurring ones. Nowadays, these are also (partly) synthetically produced using modern chemical processes . This makes no difference to the effect in the body , as these behave chemically in exactly the same way as the substances produced in the body.
Examples
Analogues are often derivatives of a biologically active basic substance, such as in the case of synthetic steroids , which are all derived from the sterane :
| derivative | structure | Surname | Substance group |
|---|---|---|---|
| Estran | 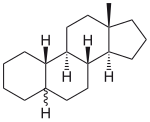 |
13β-methylgonane | Estrogen-like substances (see estrogens ) |
| Androstane |  |
10β, 13β-dimethylgonane | Androgen-like substances (see androgens ) |
| Pregnan |  |
10β, 13β-dimethyl-17β-ethylgonane | Progesterone-like substances = progestin , corticosteroid-like substances (see corticosteroids ) |
application
Analogs play a major role in combinatorial chemistry for pharmaceutical purposes, since natural substances are not patentable and only chemically “newly invented” substances are marketable on a large scale and are of interest to large corporations. In drug development , either a large number of structural analogues for a given lead structure are created and the relationship between structure and activity is tested in a study, or a database is searched for structural analogues for the given lead structure.
Examples
- GABA analogues: gabapentin , tiagabine and vigabatrin
- Pyrophosphate -A .: bisphosphonates
See also
Individual evidence
- ^ Willett, Peter, Barnard, John M., Downs, Geoffry M .: Chemical Similarity Searching . In: Journal of Chemical Information and Computer Science . 38, 1998, pp. 983-996.
- ↑ AM Johnson, GM Maggiora: Concepts and Applications of Molecular Similarity . John Willey & Sons, New York 1990, ISBN 0-471-62175-7 .
- ↑ N. Nikolova, J. Jaworska: Approaches to Measure Chemical Similarity - a Review . In: QSAR & Combinatorial Science . 22, No. 9-10, 2003, pp. 1006-1026. doi : 10.1002 / qsar.200330831 .
- ↑ a b Martin, Yvonne C., Kofron, James L. and Traphagen, Linda M .: Do Structurally Similar Molecules Have Similar Biological Activity? . In: Journal of Medicinal Chemistry . 45 (19), 2002, pp. 4350-4358. doi : 10.1021 / jm020155c .
- ↑ Schnecke, Volker and Boström, Jonas: Computational chemistry-driven decision making in lead generation . In: Drug Discovery Today . 11 (1-2), 2006, pp. 43-50. doi : 10.1016 / S1359-6446 (05) 03703-7 .
- ↑ Rester, Ulrich: From virtuality to reality - Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective . In: Current Opinion in Drug Discovery and Development . 11, No. 4, 2008, pp. 559-568. PMID 18600572 .
Web links
- Analogues in the ChEMBL, DrugBank and Connectivity Map - a web service to find structural analogues in the ChEMBL , DrugBank and Connectivity Map.