γ-aminobutyric acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
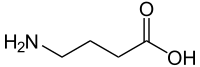
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | γ- aminobutyric acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 9 NO 2 | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 103.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
203 ° C (decomposition) |
|||||||||||||||||||||
| pK s value |
4.05 |
|||||||||||||||||||||
| solubility |
very good in water (1300 g l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
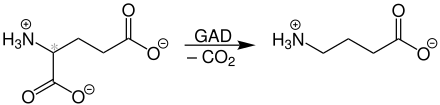
The γ -aminobutyric acid (English gamma -Aminobutyric acid , abbreviated GABA ) more rarely, 4-aminobutyric acid or piperidine acid called, is a amine of butyric acid . The position of the amino group on the γ - carbon atom with respect to the carboxy group distinguishes it from the proteinogenic α - amino acids .
Biologically, γ- aminobutyric acid or GABA is an important endogenous messenger substance in many organisms , which is formed as a biogenic amine by decarboxylation of glutamic acid , especially by nerve cells . In the brain of adult mammals GABA is the major neurotransmitter of inhibitory (inhibitory) synapses ; During the fetal maturation phase, however, the effect is often excitatory.
Binding to GABAergic macromolecules
GABA binds to specific biological macromolecules. Thus enabled it ionotropic and metabotropic GABA receptors ; it passes through membranes via plasmalemmal (GAT) and vesicular (VGAT) transporters and is a substrate for transaminase.
- GABA A receptors: The GABA receptor type A is a chloride - ion channel , via the ligand is controlled. GABA binds as orthosteric ligand to an extracellular location domain of the receptor as a transmembrane - protein the cell membrane spans and five protein subunits is composed. This binding site is at the interfaces β + / α- of the subunits; the electrophysiological properties of GABA are strongly dependent on the respective structure of the receptor type .
- The binding complex was characterized in more detail in 2018 using electron microscopy . In a predominantly stretched conformation , GABA binds with its amino group to loops B and C of the receptor subunit beta (β), namely via a salt bridge with glutamic acid (β- E 155), an aromatic cation-Pi bond (β-Y205) and a Hydrogen bond (β-Y97). The carboxy group of the ligand forms two hydrogen bonds (β-T202 and α-T129) and forms a salt bridge with the receptor subunit alpha (α) via arginine (α-R66). The ligand is stabilized in its position by surrounding aromatics (β-Y205, β-F200, β-Y157, α-F64). GABA also binds weakly to homologous binding sites α + / β-. When bound, GABA stabilizes the open conformation of the receptor, thus increasing the influx of anions and thus leading to a corresponding change in the membrane potential .
- GABA A -ρ receptors: The type once called GABA C -receptor is also an ionotropic receptor. It differs from the GABA A receptor in that it is composed of ρ subunits. Pharmacological substances such as benzodiazepines and barbiturates are ineffective at this receptor .
- GABA B receptors: This type belongs to the G protein-coupled receptors ( metabotropic ). It conveys an increased opening probability of potassium ion channels. This leads to hyperpolarization of the cell membrane. Furthermore, the probability that calcium channels will be open is reduced. This effect is mainly noticeable presynaptically , here the transmitter release is reduced.
Biosynthesis and metabolism
GABA is produced in eukaryotic cells by the decarboxylation of glutamic acid using glutamate decarboxylase (GAD). This means that an excitatory neurotransmitter can become an inhibitory one in one step.
Receptors for GABA are often found on nerve cells and usually lead to an inhibition ( inhibition ) of nerve conduction. The neurotransmitter GABA can be reabsorbed by the presynaptic neuron and stored in synaptic vesicles for reuse. Some of the GABA molecules released into the synaptic gap as transmitters are taken up by neighboring glial cells . There the amino group is transferred to pyridoxal phosphate and further to α-ketoglutarate with the help of GABA transaminase ; the resulting succinate semialdehyde is metabolized via the citric acid cycle . This metabolism, located in the mitochondrial matrix and known as the GABA byway, is not restricted to the brain, but also exists in most other organs. With the help of the transaminase inhibitor vigabatrin , this breakdown path in the brain can be inhibited. This results in an increased GABA level with a protective effect against epileptic seizures .
GABA receptors play an important role in the development of neuronal structures in the brain. Interestingly, GABA often has an excitatory effect on newly formed neuronal connections in the fetus and thus contributes to their establishment.
GABA absorbed at the periphery only crosses the blood-brain barrier in small amounts. The effectiveness of GABA as a drug has not been proven; taking it for whatever purpose is therefore not recommended.
Role of GABA in the pancreas
GABA acts as an inhibitory transmitter in the pancreas by inhibiting the glucagon secretion of the alpha cells in the islets of Langerhans . GABA, produced bacterially in the gut of overfed obese mice, improved insulin secretion and decreased the accumulation of adipose tissue in the intestinal wall.
GABA modulators
In addition to the synthetic active substance gabazine, the plant poisons picrotoxin from the myrtle and bicuculline from the heart flowers are used as GABA antagonists for basic research . Muscimol , one of the poisons of the toadstool, is relevant as a GABA agonist . The active ingredient baclofen serves as an agonist in medical applications .
See also
literature
- H. Lüllmann, K. Mohr, M. Wehling: Pharmacology and Toxicology. 15th edition, Thieme Verlag, 2003, ISBN 3-13-368515-5 .
- Klaus Aktories, Ulrich Förstermann, Franz Hofmann, Wolfgang Forth: General and special pharmacology and toxicology . 9th edition. Urban & Fischer, 2004, ISBN 3-437-42521-8 .
- Robert M. Julien: Drugs and Psychotropic Drugs. Spektrum Akademischer Verlag, 1997, ISBN 3-8274-0044-9 .
Web links
Individual evidence
- ↑ Entry on AMINOBUTYRIC ACID in the CosIng database of the EU Commission, accessed on April 18, 2020.
- ↑ a b c data sheet γ-aminobutyric acid from Sigma-Aldrich , accessed on April 26, 2011 ( PDF ).
- ↑ a b c d Entry on γ-aminobutyric acid in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ Phulera S, Zhu H, Yu J, Claxton DP, Yoder N, Yoshioka C, Gouaux E: Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA . In: Elife . July 7, 2018. doi : 10.7554 / eLife.39383 . PMID 30044221 . PMC 6086659 (free full text).
- ↑ Arne Schousbou and Helle S. Waagepetersen: Gamma-Aminobutyric Acid (GABA) in Encyclopedia of Neuroscience: Reference Module in Neuroscience and Biobehavioral Psychology 2009, pp. 511-515, doi : 10.1016 / B978-0-12-809324-5.02341- 5 .
- ↑ Michel Jung and Charles Danzin, New developments in enzyme-activated inhibitors of pyridoxal phosphate-dependent enzymes of therapeutic interest. in Design of Enzyme Inhibitors as Drugs pp. 257-293, Oxford University Press 1989.
- ↑ Lippert, B., Metcalf, BW, Jung, MJ and Casara, P .: 4-aminohex-5-enoic acid, a selective catalytic inhibitor of 4-aminobutyrate aminotransferase in mammalian brain. European Journal of Biochemistry No. 74, p. 441 (1977).
- ^ WH Oldendorf: Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection . In: Am.J. Physiology Vol. 221 (1971), No. 6, pp. 1629-1639. doi : 10.1152 / ajplegacy.1971.221.6.1629 .
- ↑ Ada McVean, GABA supplements, glorious, gimmicky or just garbage? in McGill, Office of Science and Society. Separating sense from nonsense October 11, 2018.
- ↑ Anna Wendt, Bryndis Birnir, Karsten Buschard, Jesper Gromada, Albert Salehi, Sabine Sewing, Patrik Rorsman and Matthias Braun: Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells in Diabetes No. 53 , P. 1038 (2004)
- ↑ Patrik Rorsman, Per-Olof Berggren, Krister Bokvist, Hans Ericson, Hanns Möhler, Claes-Göran Östenson and Paul A. Smith: Glucose-inhibition of glucagon secretion involves activation of GABA A-receptor chloride channels in Nature No. 341, p . 233 (1989)
- ↑ Catherine Stanton et al .: Gamma-aminobutyric acidproducing lactobacilli positively affect metabolism and depressivelike behavior in a mouse model of metabolic syndrome. In: Nature Research, Scientific Reports No. 9, 2019, p. 16323, doi : 10.1038 / s41598-019-51781-x .
- ^ W. Bautista, J. Aguilar, JE Loeza-Alcocer, R. Delgado-Lezama: Pre- and postsynaptic modulation of monosynaptic reflex by GABAA receptors on turtle spinal cord. In: The Journal of Physiology. Volume 588/14, July 2010, pp. 2621-2631, doi : 10.1113 / jphysiol.2010.188979 , PMID 20519320 , PMC 2916992 (free full text).
- ^ Makoto Taketani: Advances in Network Electrophysiology. Springer Science & Business Media, 2006, ISBN 978-0-387-25858-4 , p. 305 ( limited preview in Google book search).
- ^ BH Liu, GK Wu, R. Arbuckle, HW Tao, LI Zhang: Defining cortical frequency tuning with recurrent excitatory circuitry. In: Nature Neuroscience . Volume 10, number 12, December 2007, pp. 1594–1600, doi : 10.1038 / nn2012 , PMID 17994013 , PMC 2447868 (free full text).