Aminotransferases
| Aminotransferases | ||
|---|---|---|

|
||
| Ribbon model of aspartate transaminase according to PDB 1AAM | ||
| Enzyme classification | ||
| EC, category | 2.6.1.- , transferases | |
| Response type | Transfer of amino groups ( transamination ) | |
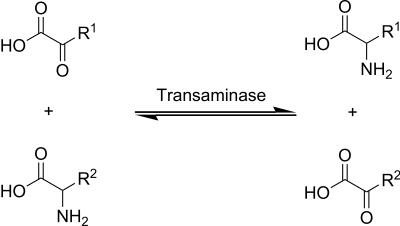
Aminotransferases or transaminases are enzymes in all living things that catalyze the transfer of α- amino groups from a donor to an acceptor molecule ( transamination ).
properties
In a medical laboratory examination for transaminases is the measurement of the blood levels for the aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) , the diagnostic importance in liver diseases have ( transaminase ) .
Transaminases are essential in amino acid metabolism and in hexosamine biosynthesis . In addition, a glutamate-1-semialdehyde aminotransferase is involved in porphyrin biosynthesis in bacteria and archaea . Transaminases are inhibited by transaminase inhibitors.
Catalyzed reaction
The donor is usually an amino acid and the acceptor is an α- keto acid , the amino acid becoming a new α-keto acid and the original α-keto acid becoming a new amino acid. In many cases, pyridoxal phosphate is involved as a cofactor .
Transaminases are available for 19 of the 20 proteinogenic amino acids, which means that these are actually only partially essential. They can be converted from special keto acids, some of which, however, are not provided by the metabolism. The partner amino acid is usually glutamate , which is deaminated to α- ketoglutarate and thus serves as a universal donor for amino groups. Therefore, glutamate is usually left out of the naming of the transaminase.
literature
- Ulf Dettmer, Malte Folkerts, Eva Kächler, Andreas Sönnichsen: Intensive course in biochemistry (Elsevier-Verlag, Munich, 1st edition 2005, ISBN 978-3437444500 )
- Smith DM, Thomas NR, Gani D: A comparison of pyridoxal 5'-phosphate dependent decarboxylase and transaminase enzymes at a molecular level . In: Experientia . 47, No. 11-12, December 1991, pp. 1104-18. PMID 1765122 .
- Rossi F, Schwarcz R, Rizzi M: Curiosity to kill the KAT (kynurenine aminotransferase): structural insights into brain kynurenic acid synthesis . In: Curr. Opin. Struct. Biol . 18, No. 6, December 2008, pp. 748-55. doi : 10.1016 / j.sbi.2008.09.009 . PMID 18950711 .
- Yonaha K, Nishie M, Aibara S: The primary structure of omega-amino acid: pyruvate aminotransferase . In: J. Biol. Chem. . 267, No. 18, June 1992, pp. 12506-10. PMID 1618757 .