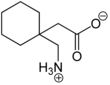
Gabapentin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Gabapentin | |||||||||||||||||||||
| other names |
2- (1- (aminomethyl) cyclohexyl) acetic acid |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass |
|
|||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
162-166 ° C and 165-167 ° C, respectively; 122–123 ° C (hydrochloride) |
|||||||||||||||||||||
| pK s value |
3.68; 10.70 |
|||||||||||||||||||||
| solubility |
slightly soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Gabapentin is a drug from the group of anticonvulsant drugs , which for the treatment of epilepsy and neuropathic pain is used. The drug was patented by Gödecke and Warner-Lambert in 1976 .
pharmacology
application areas
Gabapentin is approved for the monotherapy of simple and complex partial seizures with and without secondary generalization and for the adjunctive therapy of partial seizures with and without secondary generalization. Another indication is the treatment of neuropathic pain. Neuropathic pain occurs e.g. B. in some of the patients with shingles after the skin changes have subsided, the post-zoster neuralgia . Another common area of application is diabetic polyneuropathy and the treatment of phantom pain . Gabapentin can also be used to reduce postoperative pain. The substance can also work for a refractory cough if the cause does not require other measures.
In the context of off-label use (i.e. outside of the use approved in the approval), it can also be used for spasticity in multiple sclerosis , if the approved substances with appropriate dosage and duration of use did not provide sufficient relief or if there is intolerance. A resolution of the Federal Joint Committee (G-BA) on the prescribability in an unauthorized area of application came into force in March 2014.
Mechanism of action
The mechanism of action of gabapentin is not fully understood. Its anticonvulsant effects have not been associated with direct activation of GABA receptors , although it is structurally related to GABA. The mechanism of action discussed is an inhibition of glutamatergic transmission and (as a calcium channel blocker ) the blockade of central calcium channels (calcium channel alpha-2-delta ligand) (N, P / Q).
Side effects
The most common side effects during the use of gabapentin are pronounced dry mouth , tiredness or sleepiness , dizziness , headache, nausea, vomiting, weight gain, nervousness, insomnia, ataxia , eye tremors , paresthesia , increased appetite, but also loss of appetite and anorexia . In addition, edema, can otitis media , viral and respiratory infections , acute pancreatitis , leukopenia and thrombocytopenia , but also psychological abnormalities such as anxiety, depression , hallucinations , thought disorder , hostility, amnesia and confusion occur.
Interactions
The absorption of gabapentin can be influenced by the simultaneous intake of calcium or magnesium containing antacids . Morphine and alcohol can increase the effects and side effects of gabapentin.
Gabapentin can have a false positive effect on the results of some urine tests for protein .
Contraindications
In conduction disturbances of the heart Gabapentin is contraindicated .
Pharmacokinetics
The drug is taken orally, the active ingredient remains in the blood with a half-life of 5 to 7 hours. It is excreted in the urine.
While gabapentin is excreted unchanged in humans, metabolism to N- methyl gabapentin takes place in dogs , which is why dogs are eliminated more quickly and therefore less effective.
physical and chemical properties
Gabapentin occurs in three polymorphic crystal forms (Forms II, III and IV) and a hemihydrate form (Form I). Form II is the thermodynamically stable form at room temperature .
Gabapentin is an analogue of γ-aminobutyric acid (GABA). It is mainly present as an "inner salt" or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value), at which the gabapentin also has its lowest solubility in water. The isoelectric point is 7.14.
The compound is prone to intramolecular lactam formation. As an impurity resulting from manufacture or storage, the lactam should be avoided in the pharmaceutical product as it is more toxic than gabapentin; it creates cramps.
synthesis
Gabapentin can be prepared from diethyl 2-cyclohexylidenemalonate in three reaction steps. The 2-cyclohexyl-2-cyanomalonic acid diethyl ester is initially obtained by reaction with cyanide. Subsequent reductive cyclization produces a lactam intermediate which is hydrolyzed and decarboxylated to gabapentin under acidic conditions.
Analytics
For the reliable qualitative and quantitative determination of gabapentin, after appropriate sample preparation, both gas chromatography and HPLC can be used, especially in combination with mass spectrometry . The analytical procedure is also suitable for the detection of the contamination of water as well as for detection in forensic research. For pharmacokinetic studies, urine samples are also used to determine gabapentin.
Trade names
Gababurg (A), Gabagamma (D), GabaLich (D), Gabalster (A), Gabatal (A), Gabantin (CH), Gabax (D), Neurontin (D, A, CH), numerous generics (D, A , CH)
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 742.
- ↑ a b c d Entry on gabapentin. In: Römpp Online . Georg Thieme Verlag, accessed on July 2, 2019.
- ↑ a b Gabapentin data sheet at Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ O. Mathiesen, p Møiniche, JB Dahl: gabapentin and postoperative pain: a qualitative and quantitative systematic review, with focus on procedure. In: BMC Anesthesiol. 7, 2007, p. 6. PMID 17617920 , doi: 10.1186 / 1471-2253-7-6 .
- ^ NM Ryan: Gabapentin for refractory chronic cough. In: Lancet. 380 (9853), Nov 3, 2012, pp. 1583-1589. doi: 10.1016 / S0140-6736 (12) 60776-4 .
- ↑ Drugs Directive / Annex VI: Gabapentin for the treatment of spasticity in the context of multiple sclerosis , G-BA decision of March 28, 2014, accessed on March 29, 2014.
- ↑ Red List - Online ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ^ Specialist information Gabapentin ( Memento from November 30, 2016 in the Internet Archive ).
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 172 f.
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 172 f.
- ↑ LL Radulovic, D. Türck, A. von Hodenberg et al .: Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. In: Drug Metab Dispos. 23 (4), 1995, pp. 441-448.
- ↑ D. Braga, F. Grepioni, L. Maini, K. Rubini, M. Polito, R. Brescello, L. Cotarca, MT Duarte, V. Andre, MFM Piedade: Polymorphic gabapentin: thermal behavior, reactivity and interconversion of forms in solution and solid-state. In: New J. Chem. 32, 2008, pp. 1788-1795, doi: 10.1039 / B809662G .
- ↑ JA Ibers: Gabapentin and gabapentin monohydrate. In: Acta Cryst. C, Cryst. Struct. Comm. 57, 2001, pp. 641-643, doi: 10.1107 / S0108270101003341 .
- ↑ a b H. A. Reece, DC Levendis: Polymorphs of gabapentin. In: Acta Cryst. C, Cryst. Struct. Comm. 64, 2008, p. o105 – o108, doi: 10.1107 / S0108270107066279 .
- ↑ B. Ciavarella, A. Cupta, VA Sayeed, MA Khan, PJ Faustino: Development and application of a validated HPLC method for the determination of gabapentin and its major degradation impurity in drug product. In: J. Pharm. Biomed. Anal. 43, 2007, pp. 1647-1653. doi: 10.1016 / j.jpba.2006.12.020 .
- ↑ Cheng-Hung Hsua, Shan-Yang Li: Rapid examination of the kinetic process of intramolecular lactamization of gabapentin using DSC-FTIR. In: Thermochim. Acta. 486, 2009, pp. 5-10. doi: 10.1016 / j.tca.2008.12.008 .
- ↑ H. Potschka, TJ Feuerstein, W. Löscher: Gabapentin-lactam, a close analogue of the anticonvulsant gabapentin, exerts convulsant activity in amygdala kindled rats. In: Arch. Pharmacol. 361, 2000, pp. 200-265. doi: 10.1007 / s002109900174
- ↑ G. Griffiths, H. Mettler, LS Mills, F. Previdoli: Novel Syntheses of Gabapentin via Addition of Hydrocyanic Acid to Cyclohexylidenemalonate or Cyano (cyclohexylidene) acetate. In: Helv. Chim. Acta. 74, 1991, pp. 309-314. doi: 10.1002 / hlca.19910740208 .
- ↑ Gambelunghe C, Mariucci G, Tantucci M, Ambrosini MV: Gas chromatography-tandem mass spectrometry analysis of gabapentin in serum. ; Biomed Chromatogr. 2005 Jan; 19 (1): 63-7, PMID 15470697
- ↑ Chahbouni A, Sinjewel A, den Burger JC, Vos RM, Wilhelm AJ, Veldkamp AI, Swart EL: Rapid quantification of gabapentin, pregabalin, and vigabatrin in human serum by ultraperformance liquid chromatography with mass-spectrometric detection. , Ther Drug Monit. 2013 Feb; 35 (1): 48-53, PMID 23188183
- ↑ Brieudes V, Lardy-Fontan S, Vaslin-Reimann S, Budzinski H, Lalere B: Development of a multi-residue method for scrutinizing psychotropic compounds in natural waters. , J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Mar 15; 1047: 160-172, PMID 27436277
- ↑ Tharp AM, Hobron K, Wright T: Gabapentin-related Deaths: Patterns of Abuse and Postmortem Levels. , J Forensic Sci. 2019 Jul; 64 (4): 1105-1111, PMID 30731020
- ↑ Sim J, Kim E, Yang W, Woo S, In S: An LC-MS / MS method for the simultaneous determination of 15 antipsychotics and two metabolites in hair and its application to rat hair. , Forensic Sci Int. 2017 May; 274: 91-98, PMID 28111036
- ↑ Merrigan S, Johnson-Davis KL: Liquid Chromatography-Tandem Mass Spectrometry (LC-MS / MS) Method to Quantify Gabapentin and Pregabalin in Urine. , Methods Mol Biol. 2019; 1872: 119-127, PMID 30350285