Hybridoma technique
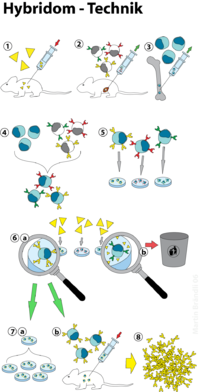
The hybridoma technology (also: hybridoma technology or hybridoma technology) is a process for the production of monoclonal antibodies (mAB). It was developed in 1975 by César Milstein and Georges Köhler , for which both researchers received the Nobel Prize in Medicine in 1984 .
In the hybridoma technique, antibody-producing cells ( B cells ) are fused with myeloma cells ( cancer cells ) , resulting in quasi-immortal hybrids that produce monoclonal antibodies. Since B cells have a limited lifespan, efficient cultivation under laboratory conditions is not possible. Myeloma cells, for their part, are highly deregulated cells that are not subject to programmed cell death ( apoptosis ). By fusing both cell types, their properties can be combined in the form of so-called hybridoma cells. These are now able to secrete monoclonal antibodies without restriction . This technique made it possible to produce antibodies in large quantities and with tailored specificity . The main areas of application of monoclonal antibodies are in use as pharmaceuticals, in diagnostics and in research. The following flow chart summarizes the processes involved in the hybridoma technique (see also Figure 1):
- Immunization (e.g .: mouse )
- Cultivation of myeloma cells
- Obtaining the B cells ( spleen )
- Cell fusion
- Selection of hybridoma cells (HAT selection)
- Screening for competent, antibody-secreting cells ( ELISA )
immunization
The first step in obtaining hybridoma cells is the immunization of donor organisms in order to obtain suitable B cells from them . Mice inbred lines (BALB / c line) that have been bred in-house are usually used for this purpose, as these are hardly prone to disease. Human, rabbit, rat and goat systems are also available. During the immunization, the donor organism is usually repeatedly administered small doses of an antigen ( vaccine ). As a result of the natural immune response , more B cells are formed that secrete antibodies against this antigen. The exact course of an immunization depends on the organism present and the antigen to be administered and can often only be determined empirically. A guideline for the immunization of mice is z. B. the administration of 100 µg antigen and 300 µl PBS buffer . To increase the chances of success, three or more animals are usually used at the same time. The spleen is removed from the animal four days after the last immunization, as a particularly large number of B cells accumulate in this organ. This is what is ultimately obtained by density gradient centrifugation . At the same time, myeloma cells that have lost their cell-specific functions (e.g. the production of antibodies) are grown in cell cultures. Cancer cells of the plasma cells have proven to be particularly suitable , since apoptotic signals are completely overridden here. After the cell fusion, they only ensure the unrestricted proliferation of the hybridoma cells. It has also proven advantageous to use myeloma and B cells from the same organism, since this increases the stability of the hybridoma cells.
Cell fusion
Basically, two methods of cell fusion have become established: (1) the action of chemical substances ( polyethylene glycol ; PEG) or (2) treatment using electrical voltage ( electrofusion ). In the first method, B cells and myeloma cells are put together in the fusion solution and centrifuged . Since the water present is largely bound by polyethylene glycol, the cell membranes are brought into close contact with one another, which leads to a spontaneous fusion of the cell membranes. Since PEG has a toxic effect on cells, the concentration and exposure time of the PEG solution are of decisive importance for the success of the fusion. In electrofusion, the cell membrane is locally "melted" by means of electrical pulses (cf. electroporation ), which causes the cell membranes to fuse. To support the fusion, PEG is usually added in small amounts here as well. After the fusion, cells that have two or more cell nuclei ( heterokaryon ) are created. In order to become an intact hybridoma cell, these nuclei must fuse spontaneously. Since chromosomes are often shed during this process , only a very small proportion of the hybridoma cells remain stable.
Selection of hybridoma cells
After the cell fusion, five cell types are present in the solution:
- Hybridoma cells
- unfused spleen cells
- unfused myeloma cells
- fused spleen cells
- fused myeloma cells
In order to select hybridoma cells that actually produce (monoclonal) antibodies, a selective medium is used in which only hybridoma cells are able to survive. A suitable medium is the so-called HAT medium, which contains the chemical substances hypoxanthine , aminopterin and thymidine . Hypoxanthine is a precursor for essential molecules ( purines ) that are required for the construction of deoxyribonucleic acid (DNA). However, the enzyme HGPRT (hypoxanthine guanine phosphoribosyl transferase) is necessary for its conversion . Since a myeloma cell line is used in the fusion that lacks this enzyme or is in an inactive form, individual myeloma cells are therefore unable to survive in this medium. Spleen cells, on the other hand, have HGPRT, but are not able to survive by themselves and die quickly in the culture medium. Only hybridoma cells can be cultivated because they have the immortality of the myeloma cells and the HGPRT genes of the spleen cells. Aminopterin only serves to block other purine synthesis pathways. Since this means that the essential thymine can no longer be produced de novo , it must be added to the medium (see also pyrimidine de novo synthesis ). However, from the hybridoma cells selected in this way, those clones which produce the desired antibodies must still be isolated ( screening ). This is preferably done using an ELISA (enzyme-linked immunosorbant assay) test. The antibodies are incubated with the antigen against which they should actually be directed. A match is proven by an enzymatic color reaction. If after a HAT selection you get e.g. For example, several tens of thousands of clones, it is not uncommon for only a few hundred cells to produce the desired antibodies. After further cultivation, only a few dozen hybridoma cells often remain stable. Some of the positive clones are stored in liquid nitrogen for later use , while the remaining cells are cultivated further. These hybridoma cultures achieve yields of up to 1 mg mAb / ml.
literature
- AE Campbell: Monoclonal antibody technology. Elsevier, 1987, ISBN 0-444-80592-3 .
- William Davis: Monoclonal antibody protocols . Humana Press, 1995, ISBN 0-89603-308-2 .
- Peter Bösch: Case study of monoclonal antibody production. Master thesis Univ. for soil culture, Vienna 2007.
- G. Köhler, C. Milstein: Continuous cultures of fused cells secreting antibody of predefined specificity. In: Nature. Volume 256, 1975, pp. 495-497.
- S. Shirahata, Y. Katakura, K. Teruya: Cell hybridization, hybridomas, and human hybridomas. In: Methods in cell biology. Volume 57, 1998, pp. 111-145.
- SP Cole, BG Campling, T. Atlaw, D. Kozbor, JC Roder: Human monoclonal antibodies. In: Molecular and cellular biochemistry. Volume 62, 1984, pp. 109-120.
- MR Clark: Monoclonal antibodies derived from hybrid myelomas. In: La Ricerca in clinica e in laboratorio. Volume 11 (3), 1981, pp. 195-203.