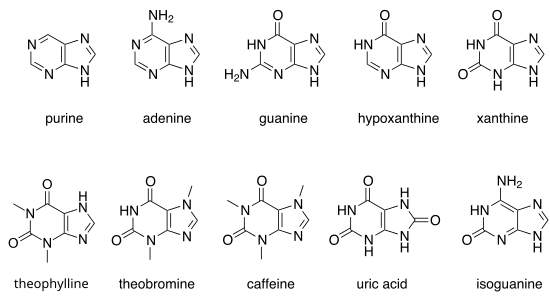
Purines
In chemistry, the purines form a group of organic compounds that belong to the heterocycles (more precisely: heteroaromatics ). They are derived from the parent compound purine .
In addition to pyrimidines, purines are important building blocks of nucleic acids . They are not essential , but are formed by the human body itself. Food of animal origin contains many purines because of their high concentrations in the skin and offal. After consumption, the purines are broken down into uric acid in humans and excreted via the kidneys ; In various other animal species, further degradation takes place (for example to allantoin in cattle ). Hence the name is derived from the Latin purus = pure and acidum uricum = uric acid, as it is the “pure” basic structure of uric acid, which was first synthesized in 1898 by Emil Fischer .
Overview
If the hydrogen atoms in positions 2, 6 and 8 are replaced by other radicals, different substituted purines result:
| Surname | Basic structure | R 6 | R 2 | R 8 |
|---|---|---|---|---|
| Purine |  |
-H | -H | -H |
| Adenine | -NH 2 | -H | -H | |
| Guanine | -OH | -NH 2 | -H | |
| uric acid | -OH | -OH | -OH | |
| Hypoxanthine | -OH | -H | -H | |
| 6-purine thiol | –SH | -H | -H | |
| 6-thioguanine | –SH | -NH 2 | -H | |
| Xanthine | -OH | -OH | -H | |
| Isoguanine | -NH 2 | -OH | -H |
All purines that occur as normal building blocks in DNA and RNA , i.e. adenine ( 2 ) and guanine ( 3 ), are referred to as purine bases . When modified, it can happen that hypoxanthine and xanthine are also temporarily part of the RNA or DNA, but they are replaced again by repair enzymes. 7-methylguanine is part of the cap structure .
Other important purines are hypoxanthine ( 4 ), xanthine ( 5 ), theobromine ( 6 ), caffeine ( 7 ), uric acid ( 8 ) and isoguanine ( 9 ).
Tautomerism
Due to the molecule segments with the basic pattern N = C – X – H (with X = O, S or NH) there is the possibility of tautomerism (see lactam-lactim , thiolactam-thiolactim and imine-enamine tautomerism ):
Biological importance
- If adenine and guanine are linked in position 9 to the C-1 atom of ribose (in DNA with deoxyribose ), the nucleosides adenosine and guanosine result . The esterification of the ribose with phosphate produces the nucleotides , which are the building blocks of numerous physiologically important molecules. (See also: Riboflavin and AMP , ADP , ATP , RNA , DNA , cAMP , NADPH , NADH , FAD , Coenzyme A , Succinyl-Coenzyme A. )
- Nitrous acid (HNO 2 ) converts the amino group into a hydroxyl group . This creates hypoxanthine from adenine and xanthine from guanine . Acting nitrous acid to the DNA (as a mutagen , performs this change to that in the multiplication of DNA () a reduplication ) to erroneous base pairing and thus to a modified base sequence comes to modified proteins, and thus an altered phenotype may result .
- The tRNA also contains unusual purine bases (long bases): A + is created by the addition of a proton at position 7, which means that the nitrogen atom at this point is positively charged. The nitrogen in the ring system can be methylated, which means that they are also positively charged. Examples: 1-methyladenine (m 1 A), 7-methyladenine (m 7 A), 7-methylguanine (m 7 G). With 2'-O-methylguanosine (m 2 ' G) the C-2 atom of the ribose is methylated. The corresponding bases are only modified after the transcription and influence the accuracy of the translation as well as the activity and stability of the tRNA (see also nucleosides ).
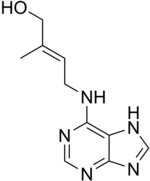
- trans -zeatin (N 6 - (Δ 2 ' -Isopentenyl) -aminopurine) is a naturally occurring cytokinin .
- The uric acid is the breakdown product of purine metabolism.
metabolism
The purine nucleotides AMP, ADP, ATP, GTP, GDP and GMP are derived from the purine heterocycle, and their common precursor in biosynthesis is inosine monophosphate (IMP). Many other substances serve as intermediate products and form a network in which each of the nucleotides can be produced from one of the intermediate products, synthesized from scratch ( de-novo ) or broken down into uric acid (see figure).
De novo biosynthesis of IMP
The purines are not synthesized in the organism as free molecules , but always as nucleotides . The starting molecule is the α- D - ribose -5-phosphate, an intermediate product of the pentose phosphate cycle . The basic structure of the purine is then built up step by step, with different molecules supplying the individual components. The end product of this synthesis chain is inosine monophosphate (IMP), the nucleotide of hypoxanthine, which is converted in further steps to the nucleotides of adenosine or guanosine.
Interconversion of AMP and IMP
AMP biosynthesis (de novo)
The synthesis of AMP starts with that of IMP and requires two steps in all living beings:
IMP and aspartate are ligated using GTP, catalyzed by adenylosuccinate synthase . Fumarate is split off from the intermediate product using adenylosuccinate lyase , resulting in AMP.
IMP from AMP
The AMP deaminase enzyme facilitates the conversion of AMP to IMP. This makes it possible for eukaryotes to obtain IMP from purine bases via the salvage route.
AMP is converted to IMP; Water is consumed, ammonia is produced.
Interconversion of GMP / XMP and IMP
GMP biosynthesis (de novo)
As with ATP synthesis, GMP is obtained from IMP in two steps. The first step, the oxidation of IMP and XMP, is catalyzed by IMP dehydrogenase .
IMP is oxidized to XMP. The second step is done using GMP synthase .
 +
+  + ATP + H 2 O + + AMP + PP i
+ ATP + H 2 O + + AMP + PP i 
![]()
GMP, AMP and glutamic acid are formed from XMP, ATP and glutamine.
IMP from GMP
The reverse reaction of IMP from GMP in one step is possible using GMP reductase . This contributes to the AMP / GMP balance in the cell.
Ammonia is split off from GMP and IMP is created. The reaction is irreversible.
Recycling of the bases (salvage pathway)
When the RNA is broken down, free bases and nucleosides are also formed in addition to mononucleotides . The mononucleotides can be recovered in that the purine bases with phosphorylated ribose and the nucleosides by kinases regain their phosphate group.
Dismantling
Phosphate is separated from the nucleotides (with the help of the enzyme nucleotidase ) and the bases are then split off from the resulting nucleosides, catalyzed by the purine nucleosidase . Guanine is deaminated to xanthine using guanine deaminase . Xanthine is oxidized to uric acid. In land reptiles , birds , many insects and primates, this is the end product that is excreted in the urine . Other animals and plants turn uric acid into allantoin , urea or ammonia , which is reabsorbed as ammonium when nitrogen is required .
Receptors
Purines bind to specific receptors in the cell membrane , so-called purinergic receptors . There are ionotropic and metabotropic purinergic receptors. The physiological agonist of these receptors is ATP .
Medical importance
Diseases
Depending on the location of the disturbance in the purine metabolism, different clinical pictures arise:
- The Lesch-Nyhan syndrome is due to a deficiency of an enzyme ( hypoxanthine-guanine phosphoribosyltransferase due, HGPRT) within the recycling of bases (nucleotide salvage). As a result, the substrates 5-P-ribosyl-PP and the purines hypoxanthine and the like are increasing. Guanine, the latter also leading to an accumulation of uric acid. This causes gout , intellectual disability, and behavior problems.
- Gout is a result of hyperuricemia , an increased concentration of uric acid in the blood (387 µmol / l). Below this concentration there are enough proteins available in the blood to transport uric acid, which is sparingly soluble in water, and to prevent its precipitation . If the uric acid level is too high, this protective system is no longer sufficient and deposits occur in joints , tendon sheaths and the kidney medulla .
- SCID (severe combined immunodeficiency) is due to a 50-fold increase in dATP concentration. This disrupts the sensitive balance in the concentration of the DNA building blocks and disrupts DNA synthesis, which primarily affects the cells of the immune system (T and B cells).
- Adenylosuccinase deficiency, a rare hereditary disease that can lead to intellectual disability and is often fatal in childhood. The enzyme concerned is adenylosuccinate lyase .
Medicinal substances
Purine derivatives and purine analogs play a role as antimetabolites : Azathioprine suppresses the immune system , 8-azaguanine, 6-purinthiol and 6-thioguanine are used against certain forms of cancer , and allopurinol against gout. N -hydroxy-purine and purine- N -oxides are carcinogenic (carcinogenic).
literature
- Gerhard Heidelmann: History and definition of the disorders of the purine metabolism. In: Gerhard Heidelmann, Peter Thiele (ed.): The gout syndrome. Arthritis, nephropathy, uric acid nephrolithiasis, diabetes mellitus, hyperlipoproteinemia, obesity, hypertension, myocardial infarction, arterial occlusion. Dresden 1974, pp. 1-5.
Individual evidence
- ^ CR Hancock, JJ Brault, RL Terjung: Protecting the cellular energy state during contractions: role of AMP deaminase. In: Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. Volume 57 Suppl 10, November 2006, pp. 17-29, PMID 17242488 . (Review).
- ↑ HN Jayaram, DA Cooney et al. a .: Consequences of IMP dehydrogenase inhibition, and its relationship to cancer and apoptosis. In: Current medicinal chemistry. Volume 6, Number 7, July 1999, pp. 561-574, PMID 10390601 . (Review).
- ^ AK Werner, CP Witte: The biochemistry of nitrogen mobilization: purine ring catabolism. In: Trends in plant science. Volume 16, number 7, July 2011, pp. 381-387, doi : 10.1016 / j.tplants.2011.03.012 . PMID 21482173 . (Review).
- ↑ BB Fredholm: Adenosine receptors as drug targets. In: Experimental Cell Research . Volume 316, Number 8, May 2010, pp. 1284-1288, doi : 10.1016 / j.yexcr.2010.02.004 . PMID 20153317 . PMC 2866745 (free full text). (Review).
- ↑ RJ Torres, JG Puig: Hypoxanthine-guanine phosophoribosyltransferase (HPRT) deficiency: Lesch-Nyhan syndrome. In: Orphanet Journal of Rare Diseases. Volume 2, 2007, p. 48, doi : 10.1186 / 1750-1172-2-48 . PMID 18067674 . PMC 2234399 (free full text). (Review).
- ^ EB Gonzalez: An update on the pathology and clinical management of gouty arthritis. In: Clinical Rheumatology. Volume 31, Number 1, January 2012, pp. 13-21, doi : 10.1007 / s10067-011-1877-0 . PMID 22069122 . PMC 3249158 (free full text). (Review).
- ^ PL Riches, AF Wright, SH Ralston: Recent insights into the pathogenesis of hyperuricaemia and gout. In: Human Molecular Genetics . Volume 18, R2 October 2009, pp. R177-R184, doi : 10.1093 / hmg / ddp369 . PMID 19808794 . (Review).
- ↑ J. Chinen, WT Shearer: Advances in basic and clinical immunology in 2010. In: Journal of Allergy and Clinical Immunology . Volume 127, Number 2, February 2011, pp. 336-341, doi : 10.1016 / j.jaci.2010.11.042 . PMID 21281863 . PMC 3057129 (free full text). (Review).
- ^ EK Spiegel, RF Colman, D. Patterson: Adenylosuccinate lyase deficiency. In: Molecular Genetics and Metabolism. Volume 89, number 1-2, 2006 Sep-Oct, pp. 19-31, doi : 10.1016 / j.ymgme.2006.04.018 . PMID 16839792 . (Review).
- ↑ M. Samsel, K. Dzierzbicka: Therapeutic potential of adenosine analogues and conjugates. In: Pharmacological Reports Volume 63, Number 3, 2011, pp. 601-617, PMID 21857072 . (Review).
- ↑ T. Robak, E. Lech-Maranda et al. a .: Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. In: Current Medicinal Chemistry . Volume 13, Number 26, 2006, pp. 3165-3189, PMID 17168705 .
Web links
- MedizInfo.de Food tables for purines and uric acid
- PurinTabelle.de Food tables for purines and uric acid
- C. Müller: Gout disease - low-purine food against pain. from: UGB-Forum 2/2000, pp. 68-71, UGB-Verband, 2000