Allantoin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Allantoin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 6 N 4 O 3 | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 158.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
~ 1.7 g cm −3 |
|||||||||||||||||||||
| Melting point |
225–236 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
poor in water (5.7 g · l −1 at 25 ° C), almost insoluble in ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
In various animal species, especially in mammals, allantoin is, in addition to uric acid, a primary end product of the breakdown of nucleic acids , especially of purine bases .
history
Allantoin was discovered around 1800 by Buniva and Louis-Nicolas Vauquelin in the body fluid that fills the allantois of cows, and it takes its name from this. The compound was later isolated from the urine of young calves and examined by Friedrich Wöhler , in cooperation with Justus Liebig . These researchers first recognized the connection with uric acid , from which they obtained allantoin through oxidation with lead dioxide.
Occurrence


Allantoin is an active ingredient found in some domestic plants (especially comfrey ). Allantoin can be found primarily in black salsify , but also in wheat seedlings, soybean seedlings, rice, cauliflower, green beans and horse chestnuts . The larvae of Lucilia sericata (species of goldfly ) are used as a means of wound healing, as they very specifically eat necrotic tissue and release large amounts of allantoin.
synthesis
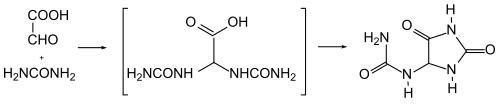
Allantoin can be obtained synthetically by heating glyoxylic acid with urea to 100 ° C. Instead of glyoxylic acid, dichloroacetic acid can be used industrially . The production from uric acid has also been optimized: Potassium permanganate in an alkaline solution (caustic soda) is used as an oxidizing agent.
use
Allantoin is used in cosmetics in skin creams, shower gels, sunscreens, aftershave lotions, toothpaste and in agents against excessive perspiration ( hyperhidrosis ) and skin irritations. It accelerates cell construction, cell formation or cell regeneration and soothes the skin. The healing of wounds that are difficult to heal is also supported, but allantoin has no antiseptic properties.
Other properties
Comfrey ointment has long been considered the best wound healing ointment in naturopathy. It was made by extracting the fat from the fresh root with lard. The active ingredient is temperature-resistant, but sensitive to contact with metals that can cause catalytic decomposition. Therefore preparations containing allantoin should not be stored in metal containers.
Individual evidence
- ↑ entry to ALLANTOIN in CosIng database of the European Commission, accessed on August 11, 2020th
- ↑ a b c d Data sheet Allantoin (PDF) from Merck , accessed on November 4, 2007.
- ↑ a b Data sheet Allantoin, ≥98.0% (N) from Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ a b entry on allantoin. In: Römpp Online . Georg Thieme Verlag, accessed on November 3, 2011.
- ↑ Buniva and Vauquelin, Annales de Chimie, Series 1, Volume 33, 269-275 (1800); quoted from Beilstein's Handbuch der Organischen Chemie, Hauptwerk, Volume 25, p. 474c and Partington, A History of Chemistry, Vol. 4, p. 333.
- ↑ F. Wöhler, J. Liebig, Annalen der Pharmazie, 26, 241-336 (1838).
- ↑ Grimaux, Comptes rendues des Seances de l'Academie des Sciences, Vol. 83, 63; Annales de Chimie, Series 5, Vol. 11, 390; quoted from Beilstein's Handbuch der Organic Chemie, Hauptwerk, Vol. 25, 474.
- ↑ CN Zellner, JR Stevens, US Patent 2158098 (1939) to Merck & Co .; Chemical Abstracts 33, 6350 (1939).
- ^ WW Hartman, EW Moffett, JB Dickey: Allantoin In: Organic Syntheses . 13, 1933, p. 1, doi : 10.15227 / orgsyn.013.0001 ; Coll. Vol. 2, 1943, pp. 21-23 ( PDF ).