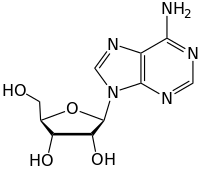
Adenosine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Adenosine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 13 N 5 O 4 | |||||||||||||||||||||
| Brief description |
white and odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 267.24 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.31 g cm −3 |
|||||||||||||||||||||
| Melting point |
234-237 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Adenosine (A, Ado) is a nucleoside and consists of the nucleic base adenine and the sugar β- D- ribose . The analogue with deoxyribose is deoxyadenosine . It is part of the energy-rich compounds ATP , ADP , AMP , ribonucleic acid (RNA), various cofactors (e.g. coenzyme A , NADPH , NADH ) and also in a neuromodulator .
properties
Adenosine is a white and odorless solid that practically does not dissolve in ethanol, but is soluble in hot water.
Biological importance
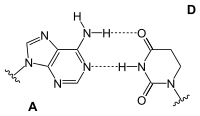
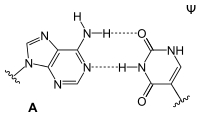
Adenosine is part of ribonucleic acid (RNA) and forms a base pair there with uridine , dihydrouridine or pseudouridine .

|
|
|
| AU base pair | AD base pair | A Ψ base pair |
Pharmaceutical importance
effect
Adenosine blocks the release of all invigorating and activating neurotransmitters such as dopamine , acetylcholine or norepinephrine . This causes dilation (widening of the blood vessels), which lowers blood pressure. Adenosine also lowers the heart rate and increases the conduction time in the AV node . This is done by activating a G i -modulated potassium channel via A 1 adenosine receptors.
Adenosine also triggers the nucleus praeopticus ventrolateralis in the hypothalamus , which inhibits the waking and waking centers of the brain through the neurotransmitter GABA , and thus has a sleep-inducing effect . The xanthines caffeine , theobromine and theophylline as well as the artificial drug istradefylline act as antagonists of various adenosine receptors . Partial inhibition of the receptors in the nucleus praeopticus ventrolateralis also contributes to their sleep-suppressing effect.
indication
Adenosine is indicated for the termination of AV nodal reentry tachycardia . It blocks the conduction of excitation from the atrium to the ventricle , causing a cardiac arrest lasting a few seconds. The physiological half-life ( half-life ) of adenosine is in the range of seconds. With theophylline one exists antidote .
Adenosine is predominantly used for the pharmacological exposure of myocardial scintigraphy .
Commercial preparations
Adenoscan (D), Adrekar (D), Krenosin (CH), Generics (D, A)
ViaSpan organ preservation solution (A), Vita-Gerin "Geistlich" (A)
Related links
- 1-methyladenosine
- 2-methyladenosine
- N 6 -methyladenosine
- N 6 cyclopentyladenosine
- N 6 -isopentenyladenosine
- N 6 -Glycinylcarbamoyladenosine
- N 6 -threonylcarbamoyladenosine
- N 6 , N 6 -dimethyladenosine
- 2'- O -methyladenosine
- 2'- O -ribosyladenosine phosphate
- Vidarabin
Web links
- Entry for adenosine in the Human Metabolome Database (HMDB) , accessed October 21, 2013.
- Modification Summary of Adenosine in the Modomics database, accessed January 13, 2014.
Individual evidence
- ↑ Entry on ADENOSINE in the CosIng database of the EU Commission, accessed on March 26, 2020.
- ↑ a b c d e data sheet adenosine (PDF) from Carl Roth , accessed on November 28, 2013.
- ↑ a b Wissenschaft-Online-Lexika: Entry on adenosine in the Lexikon der Chemie ; Retrieved November 28, 2013.
- ↑ C. Cajochen: Sleep Regulation . In: Somnologie - Schlafforschung und Schlafmedizin, Volume 13, Number 2, June 2009, pp. 64–71 ( doi : 10.1007 / s11818-009-0423-7 ).
- ↑ Wolfgang Hauber: Adenosine: a purine nucleoside with neuromodulatory effects. Neuroforum 3/02, pp. 228-234 ( PDF , 212 kB).