Istradefyllin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
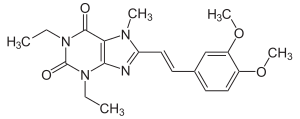
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Istradefyllin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 24 N 4 O 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Adenosine receptor antagonist |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 384.43 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Istradefylline is a drug from the group of psychotropic drugs that is being investigated for the treatment of Parkinson's disease .
properties
Istradefyllin is an antagonist at the A2A adenosine receptor . It has the longest biological half-life of any antiparkinsonian . Istradefyllin is an analogue of caffeine .
Clinical information
Application areas (indications)
Istradefyllin leads to a reduction in symptoms of the dyskinesias that occur in the classic treatment of Parkinson's disease . It reduces dyskinesias that arise from long-term treatment with classic anti-Parkinson drugs such as levodopa . The active ingredient is currently still in clinical testing, in doses of 20 mg and 40 mg per day and patient.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Istradefylline is an adenosine A 2 receptor antagonist. It thus inhibits excitatory signal transduction .
literature
synthesis
- Hockemeyer J et al. Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson's disease. J Org Chem. 2004 May 14; 69 (10): 3308-3318. PMID 15132536 .
pharmacology
- Koga K et al. Adenosine A (2A) receptor antagonists KF17837 and KW-6002 potentiate rotation induced by dopaminergic drugs in hemi-Parkinsonian rats. Eur J Pharmacol . 2000 Nov 24; 408 (3): 249-255. PMID 11090641 .
- Kase H et al. Progress in pursuit of therapeutic A2A antagonists: the adenosine A2A receptor selective antagonist KW6002: research and development toward a novel nondopaminergic therapy for Parkinson's disease. Neurology. 2003 Dec 9; 61 (11 Suppl 6): S97-100. PMID 14663020 .
application
- Bara-Jimenez W et al. Adenosine A (2A) receptor antagonist treatment of Parkinson's disease. Neurology. 2003 Aug 12; 61 (3): 293-296. PMID 12913186 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ PA LeWitt, M. Guttman, JW Tetrud, PJ Tuite, A. Mori, P. Chaikin, NM Sussman: Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson's disease: a double-blind, randomized , multicenter clinical trial (6002-US-005). In: Annals of neurology. Volume 63, Number 3, March 2008, pp. 295-302, doi : 10.1002 / ana.21315 , PMID 18306243 .
- ↑ W. Oertel, JB Schulz: Current and experimental treatments of Parkinson disease: A guide for neuroscientists. In: Journal of neurochemistry. Volume 139 Suppl 1, October 2016, pp. 325–337, doi : 10.1111 / jnc.13750 , PMID 27577098 .
- ^ T. Müller: The safety of istradefylline for the treatment of Parkinson's disease. In: Expert opinion on drug safety. Volume 14, number 5, May 2015, pp. 769-775, doi : 10.1517 / 14740338.2015.1014798 , PMID 25676023 .
- ↑ C. Zhu, G. Wang, J. Li, L. Chen, C. Wang, Y. Wang, P. Lin, H. Ran: Adenosine A2A receptor antagonist istradefylline 20 versus 40 mg / day as augmentation for Parkinson's disease: a meta-analysis. In: Neurological research. Volume 36, Number 11, November 2014, pp. 1028-1034, doi : 10.1179 / 1743132814Y.0000000375 , PMID 24725292 .