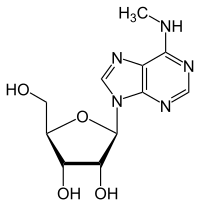
N 6 -methyladenosine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | N 6 -methyladenosine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 11 H 15 N 5 O 4 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 281.27 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
N 6 -Methyladenosine (m 6 A) is a rare nucleoside and occurs in tRNA , rRNA , mRNA and snRNA . It consists of β- D- ribofuranose (sugar) and N 6 -methyladenine . It is a derivative of adenosine , whichis methylated on the amino group .
It is generated enzymatically by methylation of adenosine by means of the N 6 adenosine methyl transferase .
The dimethylated variant is N 6 , N 6 -dimethyladenosine .
Web links
- Entry for N 6 -Methyladenosine in the Human Metabolome Database (HMDB) , accessed October 12, 2013.
- Modification Summary of N 6 -Methyladenosine in the Modomics database, accessed January 13, 2014.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Patrick A. Limbach, Pamela F. Crain, James A. McCloskey: Summary: the modified nucleosides of RNA . In: Nucleic Acids Research , 22, 1994. Number 12, pp. 2183-2196 doi : 10.1093 / nar / 22.12.2183 , PMID 7518580 , PMC 523672 (free full text).