DNA methylation
| Parent |
|
epigenetic regulation of gene expression methylation of macromolecules DNA modification |
| Gene Ontology |
|---|
| QuickGO |
When DNA methylation is a chemical modification to basic building blocks of the genetic material of a cell . This change (modification) is brought about by the transfer of methyl groups by enzymes ( DNA methyltransferases ) to nucleobases at certain points in the DNA . Since the basic structure of the respective nucleobase is retained, DNA methylation is not a genetic mutation , but a modification.
DNA methylation occurs in very many different - possibly in all - living things and has different biological functions. The sequence of DNA methylation can be based on the corresponding pattern of the mother cell and is then part of the epigenetic code of a cell. DNA methylation is the most important epigenetic change.
Occurrence
Groups of organisms
DNA methylation is found in organisms from all three domains . The top category for the division of living beings according to Carl R. Woese is used here as the domain . DNA methylations are found in the two domains of living things without a real nucleus, bacteria and archaea , as well as in the domain of living things with a nucleus , the eukaryotes .
DNA methylation not only affects the own genome of the respective cell, but can also affect other genes, e.g. B. concern that of viruses . In addition, the DNA methylation of these viruses can also affect the genetic material of the host cells (e.g. in plants, humans or bacteria).
Nucleobases
So far (2016) two nucleobases where natural, enzymatic DNA methylation takes place have been found: adenine and cytosine . The changed bases are N 6 -methyladenine , 5-methylcytosine and N 4 -methylcytosine .
| basic forms |

|

|
||||||
| Adenine, A. | Cytosine, C. | |||||||
| Altered nucleobases |

|

|

|
|||||
| N 6 -methyladenine, 6mA | 5-methylcytosine, 5mC | N 4 -methylcytosine, 4mC | ||||||

In some prokaryotic organisms, all three previously known DNA methylation types are represented ( N4-methylcytosine : m4C, 5-methylcytosine : m5C and N6-methyladenine : m6A). Six examples are shown here, two of which belong to the Archaea domain and four of which belong to the Bacteria domain . The information comes from Blow et al. (2016).
In the left column are the species names of the organisms, to the right there are examples of methylated DNA motifs.
The full name of Archaeen- or bacterial strains loud by NCBI - taxonomy :
" Methanocaldococcus jannaschii DSM 2661 ", " Methanocorpusculum labreanum Z ", " Clostridium perfringens ATCC 13127 ", " Geopsychrobacter electrodiphilus DSM 16401 ", " Rhodopseudomonas palustris CGA009 " and " Salmonella enterica subsp. enterica serovar Paratyphi A str. ATCC 9150 ".
All three variants can be found in both prokaryote domains, the bacteria and the archaea . 5-methylcytosine is often present in eukaryotes , which then occurs at CpG sites . However, N 6 -methyladenine also occurs and was first found in some unicellular eukaryotes. This concerns z. B. the green alga Chlamydomonas reinhardii and the ciliate Tetrahymena pyriformis . The presence of N 6 -methyladenine in the DNA of the mitochondria of mammals and the chromosomes was almost excluded.
Recent studies show that N 6 -methyladenine as a modified base of DNA plays a greater role in eukaryotes than previously assumed. In the nematode Caenorhabditis elegans and in the fruit fly Drosophila melanogaster , for example, N6-methyladenine is present, but 5-methylcytosine is hardly or not at all.
Luo et al. (2015) compare the occurrence of N 6 -methyladenine and 5-methylcytosine in eukaryotes and made a heterogeneous distribution of DNA methylation visible, which is little linked to the relationships; There are species that have both N 6 -methyladenine and 5-methylcytosine and many in which only one of these two modified bases can be found in the DNA.
Classification as an epigenetic modification
The eukaryotes have a nucleus with real chromosomes . They have histones that, together with DNA, make up chromatin . The DNA methylations are in close interaction with the histone modifications and the chromatin structure (e.g. the packing density of the chromosomes). The interaction of DNA methylation patterns, histone modifications and chromatin structure is a central component of epigenetics . The other two domains, bacteria and archaea, are prokaryotes . That means they have no nucleus and no real chromosomes. Prokaryotes have a nucleus equivalent that shows DNA methylation patterns but no histones.
The epigenetics is a dynamic branch of science to partially heritable phenomena in animals with cell nucleus is focused ( eukaryotes ), which are not coupled directly to the DNA sequence. Depending on how strictly epigenetics is defined, the methylations of DNA can be assigned to the epigenetic states in cells (the epigenomes ).
An essential fact that gives rise to the understanding of DNA methylation in bacteria as epigenetic changes is the discovery of the inheritance of methylation states in DNA. This inheritance was first found in a pathogenic Escherichia coli bacterium that can cause kidney inflammation. In combination with many other findings in bacteria that show similarities with epigenetics in eukaryotes, we speak of bacterial epigenetics. However, there are significant differences between bacteria and eukaryotes (cell nucleus and histones, see above), which is why the term bacterial epigenetics is used cautiously. For example, Forde et al. (2015) uses the term methylome (for the entirety of DNA methylation of a genome) without designating the methylome as part of an epigenome.
In Ee et al. (2016), the term methylome is also preferred, although the analysis of the base modifications is referred to as epigenomic analysis.
So far, we have mainly spoken of organisms with a cell nucleus ( domain eukaryota ) and the "real" bacteria (domain Bacteria ). The organisms of the third domain, the archaea ( archaea , "ancient bacteria"), are increasingly the subject of research, as modern sequencing and analysis methods allow the comparative determination of methylomes. Blow et al. (2016) were able to analyze the methylomas of 230 prokaryotes (organisms without a nucleus), in addition to 217 bacteria (Bacteria) also 13 archaea. DNA methylation was found in 93% of the sequenced organisms. The authors examined their DNA methylation patterns from different aspects and, in the synopsis of their results, speak of an "epigenomic landscape of prokaryotes" - with regard to the different binding specificities of methyltransferases, restriction endonuclease methyltransferase systems, "orphaned" methyltransferases [without reastriction endonuclease as a partner ] and gene regulatory activities. It was noticed that DNA methylation without assigned restriction systems is widespread in prokaryotes. The methylation patterns are also evolutionarily conserved, which suggests that methylation plays a role in genome regulation.
DNA methylation in bacteria
Adenine methylation plays an important role in correcting errors in freshly replicated DNA, especially in bacteria. Within GATC tetramers, the adenine is methylated at the 6-amino group (see picture on the right). Sometimes a thymine pairs with a guanine instead of a cytosine and is mistakenly incorporated when the DNA is doubled. These and other mismatches can be found by a complex that scans the DNA strand and initiates error correction (proof-reading) . The defective section in the replicated DNA strand that does not yet contain any methylated adenines is cut out. The cut piece is then replaced by a newly synthesized one. Once the proof-reading has been completed, the adenines in the new strand are methylated.
DNA methylations without associated restriction systems are widespread in prokaryotes. The methylation patterns are also conserved, ie hardly changed in evolutionary terms. This suggests that methylations play a role in genome regulation.
In a review by Casadesús and Low (2013), examples of cell differentiation in bacteria were listed that lead to different lines:
- Spore formation by Bacillus subtilis ,
- Differentiation of Rhizobium into nitrogen-fixing bactereroids,
- asymmetric cell division in Caulobacter ,
- Formation of fruiting bodies by Myxococcus ,
- Heterocyst formation in cyanobacteria and
- Formation of biofilms in many types of bacteria.
In all of these phenomena, bacterial cells with different morphological and physiological properties are formed while the genomic DNA sequence remains intact. Casadesús and Low found that long after Conrad Waddington first used the term "epigenesis", no generally accepted definition of epigenetics was agreed and favored a preliminary definition that addresses epigenetics as the study of cell line formation through non-mutational mechanisms.
In the field of bacterial epigenetics, the phase variations that contribute to the formation of pili represent examples in which the central involvement of DNA methylation has been well studied. If a methylation sequence on DNA overlaps the binding site for a protein, methylation of that sequence is blocked and alternative methylation patterns arise. For example, most GATC sites in the E. coli chromosome are fully methylated, except for a short time after DNA replication when they are hemimethylated. However, some sites are stably unmethylated due to the binding of proteins to sites that overlap or are adjacent to a GATC site and compete with Dam for binding and blocking methylation.
DNA methylation in archaea
Less is known about DNA methylation in archaea than in bacteria and eukaryotes. The technological advances of the present (2016) make this group increasingly accessible to more extensive research. According to the results obtained so far, methylation should in principle be implemented in a similar manner and have similar tasks to those of bacteria.
DNA methylation in eukaryotes
In organisms with a real cell nucleus, the eukaryotes , the methylation of the two nucleobases adenine and cytosine to N 6 -methyladenine and 5-methylcytosine is known.
Here eukaryotes can be differentiated according to whether they are
- the methylation of adenine to N6-methyladenine plays a role (e.g. the brush mold penicillum , the nematode Caenorhabditis elegans and the fruit fly Drosophila ),
- the methylation of cytosine to 5-methylcytosine is in the foreground (e.g. the plant Arabidopsis , the mold Neurospora and humans ),
- or both adenine and cytosine can be used for DNA methylation (e.g. in the green alga Chlamydomonas ).
When cytosine is used for the methylation of DNA, it is in many cases the conversion of cytosine to 5-methylcytosine within CG sequence motifs. CG methylation plays an important role in promoter inactivation, chromatin condensation, genomic imprinting and X chromosome inactivation (e.g. in Acker-Schmalwand ).
In vertebrates, CpG dinucleotides are mostly those CG sequence motifs that are subject to DNA methylation. This applies to humans and other mammals, for example. CpG islands are regions in the genome in which the CpG dinucleotides occur with particular concentration.
The distribution of the CpG dinucleotides within the human genome and the targeted, selective methylation of the cytosines are of central importance for understanding epigenetics in humans and the development of diseases.
With plants
Significant progress has been made in understanding DNA methylation in the model plant Arabidopsis thaliana . DNA methylation in plants differs from that in mammals: while DNA methylation in mammals occurs primarily at the cytosine nucleotide in a CpG site , the cytosine in plants can be methylated at CpG, CpHpG and CpHpH sites, with H a represents any nucleotide, but not guanine. Overall, the Arabidopsis DNA is highly methylated. Using mass spectrometry analyzes, the proportion of modifying cytosines was estimated to be 14%.
The main Arabidopsis DNA methyltransferase enzymes that transfer and covalently attach methyl groups to DNA are DRM2, MET1, and CMT3. Both the DRM2 and MET1 proteins share significant homology to the mammalian methyltransferases DNMT3 and DNMT1, respectively, whereas the CMT3 protein is unique to the plant kingdom.
There are currently two classes of DNA methyltransferases: 1) the de novo class of enzymes, which generate methylations on DNA new ( de novo ) and 2) a "maintenance class" of enzymes, which mark the methylations on the parent strand recognizes the DNA and after the DNA replication transfers the corresponding methylations to the respective daughter strand. DRM2 is the only enzyme that is currently considered a de novo DNA methyltransferase. DRM2, along with MET1 and CMT3, has also been shown to be involved in the maintenance of methylation markings through DNA replication. Other DNA methyltransferases are expressed in plants, but they have no known function (see chromatin database ).
It is not clear how the cell determines the sites of de novo DNA methylation, but there is evidence that many (if not all) sites involve RNA-directed DNA methylation (RdDM). In RdDM, specific RNA transcripts are made from a genomic DNA template, and this RNA forms secondary structures called double-stranded RNA molecules. The double-stranded RNAs direct the de novo DNA methylation of the original genomic region that produced this RNA; either via the small interfering RNAs (siRNAs) or via microRNAs (miRNAs). It is believed that this type of mechanism is important in cellular defense against RNA viruses and / or RNA transposons , both of which often form double-stranded RNA that can be mutagenic to the host genome. It is assumed that these RNA viruses and / or transposons are switched off by a still poorly understood mechanism with the help of methylation of the corresponding locations in the genome and are therefore no longer active in the cell, which would protect the genome from mutagenic effects.
With insects
Functional DNA methylation was discovered in honey bees . The DNA methylation markers are mainly located within genes. Current belief is that DNA methylation works in gene regulation and alternative splicing . In the fruit fly Drosophila melanogaster , the DNA methylation level for cytosine is almost undetectable. Sensitive methods applied to Drosophila DNA estimate proportions in the range of 0.1-0.3% of total cytosine. This low degree of methylation appears to be in genomic sequence patterns that are very different from the patterns observed in humans or other animal or plant species. The genomic methylation in D. melanogaster was found on specific short motifs (concentrated in specific 5-base sequence motifs that are CA and CT rich but are depleted in guanine) and is independent of DNMT2 activity. In addition, highly sensitive mass spectrometry approaches have now shown that low (0.07%) but significant adenine methylation values are present in the earliest stages of Drosophila embryogenesis .
With mushrooms
Many mushrooms have low levels (0.1-0.5%) of cytosine methylation, while other mushrooms have methylated up to 5% of the genome. This value appears to vary both between species and between isolates of the same species.
There is also evidence that DNA methylation may be involved in state-specific control of gene expression in fungi. However, with a detection limit of 250 attomoles using ultra-highly sensitive mass spectrometry, DNA methylation in single-cell yeast species such as Saccharomyces cerevisiae or Schizosaccharomyces pombe was not confirmed, which indicates that yeasts do not have this DNA modification. Although brewer's yeast ( Saccharomyces ), fission yeast ( Schizosaccharomyces ) and Aspergillus flavus show no detectable DNA methylation, the model of the filamentous fungus Neurospora crassa has a well-characterized methylation system.
Some genes control methylation in Neurospora: a mutation in DNA methyltransferase (dim-2) eliminates all DNA methylation, but does not affect growth or sexual reproduction. Although the Neurospora genome has very little “repeated DNA” (= sequence repetitions ). half of the methylation occurs in DNA that also includes transposon relics and centromere DNA. The ability to evaluate important phenomena in a DNA methylase deficient genetic background makes Neurospora an important system for studying DNA methylation.
In lower eukaryotes
The “lower cell nucleus-containing organisms” are not a phylogenetic group, that is, not a group in which there are uniform relationships. Therefore, uniformity in terms of DNA methylation is not to be expected. DNA methylation is virtually non-existent in Dictyostelium discoidium, for example, as it occurs with only about 0.006% of the cytosines. In contrast, DNA methylation is widespread in Physarum polycephalum , where 5-methylcytosine accounts for up to 8% of total cytosine
In mammals
Epigenetic changes in CpG dinucleotides
The most important epigenetic change is the methylation of cytosine as the nucleobase of DNA. In mammals, only those cytosines are methylated that are found within CG dinucleotides (also called CpG dinucleotides or CpG sites). Other cytosines are not changed by the known human DNA methyl transferases (DNMT).
During the DNA doubling before each cell division there is the old DNA strand on which certain cytosines are methylated, while the newly formed DNA strand is not yet methylated. The DNMT3 enzyme methylates each cytosine in a semi-methylated CG / CG pair. Such CG methylation means that methyl CG-recognizing proteins can bind to these me CG pairs. This binding leads to the accumulation of further proteins and to the compression of the nucleosomes (see below). As a result, the DNA on such me CG pairs cannot be read by the RNA polymerase and the gene below is inactive.
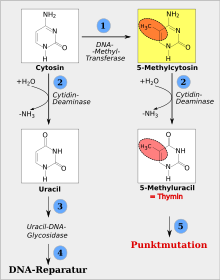
Methylated cytosines are prone to deamination , in which case the cytosine loses the amino group at position 4 of the ring. A deaminated, non-methylated cytosine is a uracil . This is not one of the four normal DNA bases adenine, cytosine, guanine or thymine. Therefore, a uracil in the DNA is recognized as a fault and replaced. However, if a 5-methylcytosine is deaminated, it creates 5-methyluracil, also known as thymine , which is a common DNA base. In a DNA double strand, the normal DNA repair apparatus cannot recognize whether the thymine or the opposite guanine is incorrectly incorporated. Therefore, the deamination of a methylcytosine into a thymine is problematic. If the conversion is preserved and has taken place in a germ cell, it can also be inherited (as a C → T point mutation ).
If you count how often pairs of two of adjacent nucleobases occur in total in the nucleotides of a nucleic acid, you can see that almost all pairs are about the same frequency. Only pairs of cytosine and guanine (CG or GC) occur much less frequently. One reason for this is believed to be that methylated cytosine which has been deaminated was not repaired, and therefore the frequency of C is lower.
The CG dinucleotides that have been preserved occur more frequently in the gene areas that are responsible for the control of genes, the promoters . Some of the promoters have a high density of CG dinucleotides, one speaks of a CpG island (cytosine phosphate guanine island). If CG pairs were converted into TG pairs, cell functions could be changed or lost. If such a change endangers the existence of the cell or the embryo, selection against the change takes place and the change is not inherited. CpG islands are generally unmethylated in healthy cells; in promoters with a low CG density there is a qualitative relationship between methylation and the activity of the associated gene. If a promoter is methylated, the controlled gene is usually inactive.
Cytosine methylations can be determined by bisulfite sequencing .
| Consequences of Deamination — Why Methylated Cytosine is a Mutation Hotspot |
|---|
The scenario and goal of the illustration
Notes on the picture elements
|
|
Illustration
The picture opposite (consequences of deamination) is explained here using five points (for details see above). (1) The nucleobase cytosine can be methylated by a DNA methyl transferase , which produces 5-methylcytosine. (2) Both cytosine and 5-methylcytosine can e.g. B. by a cytidine deaminase , be deaminated. If cytosine is deaminated on one side (left), uracil is produced; if 5-methylcytosine is deaminated on the other side (right), 5-methyluracil = thymine is formed. (3) The uracil shown on the left is non-DNA and can therefore be recognized very easily by the DNA repair apparatus. The wrong base can be cut out by uracil-DNA-glycosidase , which leads to a temporary gap in the DNA (base excision). (4) A gap created by uracil-DNA-glycosidase is mostly correctly filled with cytosine. This will restore the original condition and the result would be a successful DNA repair . (5) The thymine shown on the right, in contrast to the uracil shown on the left, is a DNA base and is therefore treated less rigorously by the DNA repair apparatus than uracil. If accepted, the DNA repair apparatus would assign a complementary guanine to the thymine in the opposite DNA strand and thus fix the conversion of cytosine to thymine (C → T transition ). The result would be a point mutation . |
DNA methyltransferases (DNMT)
So far, three human DNA methyltransferases are known: DNMT1, DNMT3a and DNMT3b (DNMT2 methylates RNA). For the Conservation methylation ( Maintenance methylation) during cell division is responsible DNMT1. DNMT3a and DNMT3b methylate the CG dimers, which are re-methylated due to cell differentiation ( de novo methylation). Mutations in the DNMT3b gene on chromosome 20 lead to immunodeficiency / centromere instability / facial expression abnormality syndrome (ICF). The methyl-binding protein ( MeCP ) can attach to methylated DNA . This in turn is the nucleus for further protein deposits, which ultimately also lead to the modification of histones. Condensed histone in cooperation with the protein complex triggered by MeCP leads to the inactivation of a chromosome segment.
DNA demethylase
The methyl-releasing enzyme DNA demethylase was also identified. It had previously been described as methyl CpG domain binding protein 2 (MBD2). Thus, the methylation of DNA is not a one-way street, but the methylation state can be regulated depending on the cell function. Such a situation is called plastic.
Embryonic development

% CpG methylation :% CpG methylation; FERTILIZATION : fertilization ; zygote : zygote ; blastocyst : blastocyst ; Epiblast : epiblast ; Somatic tissues : somatic tissues ; Germline : germline ; PGC : primordial germ cells ; BIRTH : birth ; Gametes : germ cells ; E0.5 ... E13.5 : days of embryonic development after fertilization; Ovulation : follicle rupture .
Between generations, the DNA methylation patterns in mammals are largely erased and restored. The deletion affects almost all parent methylations. This demethylation with subsequent remethylation takes place twice: first during gametogenesis and again in early embryogenesis. Demethylation in early embryogenesis occurs in two stages in the preimplantation phase - first in the zygote , then in the first embryonic replication cycles of the morula and blastula . A methylation wave then takes place during the implantation phase of the embryo, whereby the CpG islets are protected from methylation. As a result, the household genes are expressed in all cells. After that, in the post-implantation phase, the DNA methylation patterns are stage and tissue specific, that is, they show the differences that define each individual cell type and remain stable over a long period of time.
Although DNA methylation per se is not necessary for transcriptional silencing (= switching off gene activity ), it is assumed that it still represents a “blocked” state that definitely inactivates transcription. In particular, DNA methylation seems to be crucial for maintaining monoallelic silencing in the context of genomic imprinting and the inactivation of the X chromosome.
In these cases, the expressed and silent alleles differ in their methylation status, and loss of DNA methylation leads to loss of imprinting and re-expression of Xist in somatic cells. Xist (X-inactive specific transcript) is an RNA gene on the X chromosome of placental mammals that acts as the main effector of the X inactivation process. Few genes change their methylation status during embryonic development. An important exception are many genes that are specifically expressed in the germline.
DNA methylation appears to be absolutely necessary in differentiated cells, since the knock-out of one of the three effective DNA methyltransferases leads to death as an embryo or after birth. In contrast, DNA methylation can be dispensed with in undifferentiated cell types such as the inner cell mass of blastocysts , primordial germ cells or embryonic stem cells . Since DNA methylation appears to regulate only a limited number of genes directly, it remains to be seen how precisely the absence of DNA methylation causes differentiated cell death.
Although maternal and paternal genomes are reprogrammed each time they pass the germline, they are different. This phenomenon is called genomic imprinting. During gametogenesis, the DNA methylation patterns in the primordial germ cells are deleted and restored based on the sex of the sending parent. After fertilization, the paternal and maternal genomes are demethylated and remethylated again (with the exception of differentially methylated regions that are associated with imprinted genes). This reprogramming is likely necessary for the totipotency of the newly formed embryo and the erasure of acquired epigenetic changes.
The role of methylation of CpG sites on memory
In mammals, the DNA methyltransferases show a preference for the cytosines, which are located within CpG sites. In the brain of mice, 4.2% of all cytosines are methylated, with mainly 5-methylcytosine being formed at CpG sites (5mCpG). Most hypermethylated 5mCpG sites increase the suppression of associated genes.
Duke et al. describe that DNA methylation in neurons is changed by neuronal activity and that this causes the expression of certain genes to be suppressed. Neuronal DNA methylation is required for synaptic plasticity and is altered by experience; active DNA methylation and demethylation is required for memory formation and maintenance.
Halder et al. (2016) subjected Mäuse and Duke et al. (2017) subjected rats to context-dependent fear conditioning, which resulted in a particularly strong long-term memory. 24 hours after conditioning, in the rat hippocampal brain region, expression of 1048 genes was downregulated (normally associated with 5mCpG in gene promoters) and expression of 564 genes was upregulated (often associated with hypomethylation of CpG sites in gene promoters connected is). 24 hours after training, 9.2% of the genes in the rat genome of hippocampal neurons were differentially methylated. While the hippocampus is essential for learning new information, it does not store information itself. In Halder's mouse experiments, 1206 differentially methylated genes were observed in the hippocampus one hour after the context-dependent fear conditioning, but these altered methylations were restored after four weeks. In contrast to the lack of long-term CpG methylation changes in the hippocampus, a significant differential CpG methylation during memory maintenance was demonstrated in cortical neurons. Four weeks after contextual fear conditioning, there were 1223 differentially methylated genes in the anterior cingulate cortex of mice.
Biological functions of DNA methylation
DNA methylation is very widespread (see above) and therefore has a corresponding variety of biological functions, e.g. B. in the field of epigenetics . Some functional aspects of DNA methylation are discussed in more detail below:
With prokaryotes
- Protection against foreign DNA: Differentiation of the cell's own DNA from that which has entered the cell from outside.
- Correction of errors in DNA synthesis: Differentiation of the original (methylated) DNA strand from the newly synthesized strand in which the nucleobases are not yet methylated.
In eukaryotes
- Use of DNA as an information carrier: marking of active and inactive areas of the DNA, among other things depending on age.
DNA methylation and protection against foreign DNA
DNA is widespread. Cells of different types can exist in the immediate vicinity. During the ingestion of food by a cell (e.g. phagocytosis ) as well as during parasexual and sexual processes, DNA is absorbed from one (living or dead) cell into another cell. In addition, many cells are able to take up foreign DNA easily under certain circumstances ( cell competence ).
Since a living cell can only maintain its integrity if the genetic information is meaningful, it should be able to recognize and eliminate foreign DNA. This is often ensured by a system made up of two enzyme groups : the DNA methyltransferases and the restriction endonucleases .
The methyl transferases recognize a (mostly short) DNA sequence and attach a methyl group to a defined nucleobase. This creates a so-called methylation pattern. In addition, the restriction enzymes each recognize a (mostly short) DNA sequence and separate the DNA at defined points between phosphate and deoxyribose in the case of certain methylations that are present or absent. Many restriction enzymes are sensitive to methylation. This means that they only cut the DNA if there is methylation at certain points or if there is no methylation at certain points.
The system of methyl transferases and restriction enzymes in a living cell is coordinated so that its own DNA is not cut. However, foreign DNA that enters the cell under consideration from outside has a different methylation pattern in the vast majority of cases. Therefore, it is very likely to be digested by the restriction enzymes as well as other nucleases . In rare cases, foreign DNA is not or only partially digested and permanently integrated into the cell's own DNA. The integration of foreign DNA is also known as horizontal gene transfer and is a motor of evolution .
A simple methyltransferase / restriction enzyme system is explained below as an example.
Example of DNA methylation and DNA restriction
The interaction of DNA methylation and DNA restriction (cleavage of DNA) will be described using the enzymes Dpn M ( DNA methyl transferase ) and Dpn II ( restriction enzyme ). The enzymes come from the bacterium Diplococcus pneumoniae . The methyltransferase Dpn M ensures that the palindromic sequence GATC is methylated in adenosine :
m
--GATC--
--CTAG--
m
This allows "fresh DNA" that has just been created to be differentiated from the old DNA that served as a template:
m --GATC-- --CTAG--
This is important for the correct repair of errors during DNA replication . The so-called hemimethylated state (one side is methylated, the other not) is subsequently caused by the methyltransferases - such as e.g. B. Dpn M - abolished by methylation. If DNA of another species gets into the D. pneumoniae cell, this DNA is usually not methylated in the GATC sequence:
--GATC-- --CTAG--
This double-stranded DNA is very likely to be cut by the restriction enzyme Dpn II. In addition to other processes, this means that foreign DNA serves more as food and less as genetic material. This is also a mechanism that bacteria use to protect themselves from bacteriophages - by cutting the DNA into small pieces.
DNA methylation and correction of errors in new DNA synthesis
The identical duplication of deoxyribonucleic acid ( DNA replication ) is an essential prerequisite for cell division and thus for reproduction. DNA replication is ensured by the fact that enzymes (the DNA-dependent DNA polymerases) “read” the existing old strand and thereby “write” the new strand. Errors can occur. The faults can be recognized by the DNA repair systems of a cell, since there is no complementary base pairing there. However, it would remain unclear which of the two options is the right one if the old and new DNA strands do not differ. However, since the old strand is methylated but the new one is not yet, a distinction is possible. The DNA repair systems of bacteria can use this hemimethylated ("semi-methylated") state for post-replicative error correction.
In eukaryotes , the repair enzymes are z. B. by the ring clamp protein ( PCNA - Proliferating Cell Nuclear Antigen), which holds the strands apart during replication. However, there are other repair mechanisms. In the cells of humans and other mammals is a short sequence of two basic building blocks ( nucleosides ) the basis for a DNA methylation: the CpG dinucleotide (deoxy c ytidin - P hosphorsäure - deoxy g uanosin). The cytosine is methylated in the sequence of the two nucleobases cytosine-guanine. With the exception of a few areas, this occurs in almost all of the human genetic material. Here, too (as with bacteria), a hemimethylated state is crucial for post-replicative repair. In the following, such a repair is simplified using an example, shown in six steps:
|
1. Before the DNA replication, the CpG dinucleotides in both strands are methylated on the cytosine in the example section under consideration. 2. An error occurs during DNA replication: A cytidine triphosphate is used instead of a thymidine triphosphate molecule. As a result, the complementary base pairing is canceled at the corresponding point. 3. After completion of the DNA replication in the example section, the DNA double strand is hemimethylated. That is, the old strand is methylated, the new one is not. A protein complex binds to the half-side methylated CpG sites (hemi m CpG NP95-Dnmt1) and allows a subsequent repair to into an area in which the DNA double-strand starts, with the histones to chromatin to assemble . 4. The location of the mismatch is recognized. From the two possibilities adenosine monophosphate and cytidine monophosphate, the cytidine monophosphate, which is in the non-methylated strand, is cut out. Tymidine triphosphate is used to fill the gap. 5. The repaired DNA double strand is in the hemimethylated state. 6. The transfer of methyl groups to the cytosine nucleobase in the CpG dinucleotides restores the basic state. |
|
DNA methylation and the use of DNA as an information carrier
DNA methylations are markings that allow living cells to selectively use areas within the DNA for various processes. The marking of DNA can be viewed in a similar way to the formatting of text in a book: if a keyword is highlighted in a lexicon, it has a different meaning for the reader than the same word in the body text. There are several (overlapping) ways in which DNA methylations vary the type of interpretation of information that is stored in the sequence of building blocks of DNA.
DNA methylation and gene regulation
In an area in front of a gene (upstream, upstream ) there are often sites that differ from the environment in terms of their methylation pattern. In many cases, the degree of methylation can vary in different situations. This enables a selective reading frequency of the underlying gene, which is known as gene regulation or differential gene expression . Examples of such regions that can be selectively methylated are CpG islands .
DNA methylation and imprinting (genomic imprinting)
The genomic imprinting is a special case of gene regulation , which is controlled usually by DNA methylation. Different DNA methylation patterns in the male and female germ cells can be used to distinguish paternal and maternal alleles . For genes that are subject to imprinting , only the maternal or paternal allele is used. This enables a gender-specific expression of phenotypic characteristics.
Medical importance
As faulty DNA methylations at the cell level lead to reduced or increased gene activity and these changes in activity are usually inherited in a stable manner in daughter cells, they are often also the cause of diseases at the organism level. So show z. B. tumor cells often exhibit methylation patterns that differ significantly from those of healthy tissues. A tumor can arise as a result of excessive methylation (hypomethylation-hypermethylation) of upstream DNA areas, as well as a reduced degree of methylation. The regulatory area in front of each gene ( promoter area ) consists of various typical DNA sequences that represent special binding sites for different enzymes. Usually, a hypermethylated upstream DNA blocks the access of transcription-active factors and enzymes, whereby the gene activity of the following gene is suppressed.
The areas of DNA that are of particular importance for methylation are called CpG islands . Their GC content is about 60% (total genome: about 40%), and the dinucleotide cytosine-guanine (5'-CpG-3 ') is found in these sections with a frequency ten to twenty times higher than that of the rest of the genome. In human genetic research, CpG islands are often used to assign genes to genetic diseases. The genes and the areas in front of the respective gene controlled by DNA methylation can be used for the diagnosis of hereditary diseases with molecular genetic methods.
Treatment of diseases by specifically influencing DNA methylation has so far not been possible in the foreseeable future - partly because too little is known about the “correct” methylation pattern of healthy tissues. Currently there are only experimental in-vitro approaches, using so-called zinc finger proteins (a special class of proteins that have DNA-binding domains around a central zinc ion and can be coupled with methylases or demethylases) in order to be able to specifically modify certain sequences .
Regulation of DNA methylation in tumors
DNA methylation in tumor cells is different from that in healthy cells.
- The analysis of the DNA methylation of tumor cells has shown that the genes for so-called tumor suppressor proteins are often methylated in tumor cells compared to normal cells .
- In acute myeloid leukemia (AML), the CG island of the P15 protein (also called CDKN2B or ink4b) is often methylated.
- P15 is an inhibitory regulator of the cell cycle.
- After the formation of me CG in the CG island of P15, its transcription and the biosynthesis of the P15 protein are stopped.
- In the cell cycle regulator P53 , the P53 gene is hypermethylated and thus inactivated in 50% of all human tumors.
- Since P53 controls proof-reading , switching off P53 relinquishes the error control and mutations can accumulate, which can lead to the switching off of further tumor suppressor genes or to the activation of proteins that promote cell growth.
On the other hand, global DNA methylation is lower in tumor cells than in normal cells. This is attributed to the fact that the highly methylated heterochromatin in normal cells (especially the centromere region) is less methylated in tumor cells.
Since the influence of hypermethylation on tumor growth has been identified, ways have been sought to subject the developing or already existing tumors to cell cycle control by demethylation:
- Cytosine-like substances such as azacytosine or aza-deoxy-cytosine are infused into patients with acute myeloid leukemia.
- These substances are taken up in cells, whose DNA is duplicated.
- Azacytosine can be converted into aza-deoxy-cytosine in the cell.
- Aza-deoxy-cytosine is built into DNA instead of cytosine.
- The DNMT3 that wants to methylate the hemimethylated CGs binds to the aza analog.
- The exchange of carbon for nitrogen causes the enzyme to cling to the DNA during the enzymatic methyl transfer and cannot carry out any further reactions.
- This procedure inactivates and eliminates the DNMT3. Methylation no longer takes place.
- After the next cell division, the DNA is less methylated. If this de-methylation z. B. the P53 or the P15 gene are affected, cell cycle control takes place again.
- The tumor growth is thus prevented.
Clinical studies have been published in which aza-deoxy-cytosine has been shown to have an inhibitory effect on tumor development in human patients. The researchers call their procedure epigenetic therapy .
For the treatment of myelo-dysplastic syndrome , which often develops into acute myeloid leukemia, 5-aza-2'-deoxy-cytosine was approved by the FDA in 2006 as a drug under the name Dacogen . Another name for this substance is decitabine .
Differentiation of terms in connection with DNA methylation
Methylation
The methylation is a chemical modification of universal molecules . In the field of inorganic and organic chemistry, the modified molecules are also called derivatives ; in the biological analysis of macromolecules modified in this way, one speaks of modifications.
In addition to the nucleobases in the DNA, proteins can also be methylated by methyltransferases.
DNA methylation
In a narrower sense, DNA methylation refers to the natural, enzymatic transfer of methyl groups (–CH 3 ) to the nucleobases of DNA. The result of this process is also referred to as follows: the natural occurrence of methylation as chemical changes in the basic building blocks (nucleobases) of the genetic material (DNA). The DNA methylation is in principle reversible (traceable). The removal of methyl groups is called demethylation .
In a broader sense, the term "DNA methylation" is used for complex biological processes that include the creation and deletion of DNA methylation patterns (e.g., mammalian embryogenesis).
DNA demethylation
DNA demethylation refers to the change in nucleobases that were previously methylated and then no longer are. This can be the actual removal of methyl groups by appropriate enzymes ( demethylases ) so that the original nucleobases are restored ( demethylation in the narrower sense) or a conversion of the methyl groups into other chemical modifications (e.g. conversion of 5 -Methylcytosine in 5-hydroxymethylcytosine , "demethylation" in a broader sense).
Modification and mutation
The term “modification” is used with different meanings in biology . One speaks of a phenotypic modification when the properties of a living being change due to changed environmental conditions (changed phenotype ) without the genetic material being changed in the sequence of its basic components (unchanged genotype ). If the term “modification” is used without further attributes, this phenotypic modification is usually meant. DNA methylation is a modification of DNA and thus a modification of macromolecules. “DNA modification” and “phenotypic modification” are different terms, but they have one thing in common: they come about without changing the sequence of the basic building blocks of deoxyribonucleic acid. The DNA modification and the phenotypic modification are therefore not mutations . However, DNA modifications can result in mutations. This increases the probability that a cytosine is converted to thymine ( point mutation ) if this cytosine occurs methylated (see CpG sites ).
Modifications of the DNA can also result in phenotypic modifications: changed environmental conditions lead via signal transduction to an altered methylation pattern of the DNA in certain areas (DNA modification); this changes the conditions for accessing genes. This allows the use of genes to be modified (differential gene expression ); this is shown by a change in the characteristics of the living being (phenotypic modification).
A mutation is inheritable by definition. There are no DNA modifications, or only under certain conditions. One example of the partial inheritance of DNA modifications is genomic imprinting . DNA methylation is used to distinguish between paternal and maternal alleles of the same gene. In the case of imprinted genes, only one allele is active. The DNA methylation patterns responsible for this are carried over to the next generation, i.e. inherited. Since the basic building blocks (nucleobases) are retained in both alleles and the modification can be traced back through DNA demethylation, genomic imprinting is neither mutations nor genetic inheritance .
Epigenetic marking
Depending on which definition is used for epigenetics, methylations on the nucleobases of DNA are epigenetic.
The definitions most commonly used at present (2017) relate to organisms with a cell nucleus ( eukaryotes ) and use terms such as mitosis , meiosis or chromosome ; as a result, they do not include living things without a nucleus ( bacteria and archaea ). Researchers in the field of bacterial epigenetics have suggested using a tentative definition that addresses epigenetics as the study of cell line formation by non-mutational mechanisms until a generally accepted definition of epigenetics has been agreed.
Conrad Hal Waddington coined the term epigenetics . In the early 1940s he defined epigenetics as the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being - the branch of biology that studies the causal interactions between genes and their products that give rise to the phenotype. According to this original and more general definition, DNA methylations, which influence the respective phenotype, would also be epigenetic in organisms without a cell nucleus.
Without proof of the respective causal connection, the phrase "epigenetic marking" is often used to describe the fact that parts of the genetic material are marked by DNA methylation above the genetic level ( old Greek ἐπί epi 'on', 'additionally', 'also '). In this sense, DNA methylations are epigenetic markers in all living things in which they occur.
literature
- A. Jowaed, I. Schmitt, O. Kaut, U. Wüllner: Methylation regulates alpha-synuclein expression and is decreased in Parkinson's disease patients' brains . In: J. Neurosci. tape 30 , no. May 18 , 2010, p. 6355-6359 , doi : 10.1523 / JNEUROSCI.6119-09.2010 , PMID 20445061 .
Remarks
- ↑ The third methylated form of a nucleobase, N 4 -cytosine, was described by Ehrlich et al. (1985, PMID 4000939 ) found in various prokaryotes. At that time they were generally referred to as bacteria and were later assigned to the various domains Bacteria and Archaea (Woese et al. 1990, PMID 2112744 ).
- ↑ Casadesús & Low (2013, PMID 23592777 ) cites "Waddington CH (1957) The Strategy of the Genes, George Allen and Unwin, London"; the work is available as "CH Waddington: The Strategy of the Genes . In: Routledge Library Editions: 20th Century Science , Volume 20 (274 pages), May 7, 2014, ISBN 978-1-13801731-3 (hard copy)". A preview (overview of the content) can be downloaded as a GooglePreview (accessed 2019-10, https://content.taylorfrancis.com/books/download?dac=C2013-0-26528-7&isbn=9781317657552&format=googlePreviewPdf ).
- ↑ On mitosis and meiosis, Egger et al. (2004, PMID 15164071 ) stated the following: " The term epigenetics defines all meiotically and mitotically heritable changes in gene expression that are not coded in the DNA sequence itself. "And the following about the chromosome is in Bird (2007, PMID 17522671 ):" ... the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states. "
- ↑ The text with the wording "the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being" is for example in Urvalek et al. (2014, PMID 24962884 ) and by Spinelli (2017, doi : 10.21767 / 2472-1158.100077 ) in the early 1940s. Both works refer to Waddington (1968, doi : 10.1038 / 218525a0 ). Another source taken up in this context is a reprint (2012, PMID 22186258 ) of " Waddington CH (1942) The epigenotype. Endeavor 1: 18-20. ", In which the basic message of the said text is found, albeit not with the exact wording.
Individual evidence
- ^ A b D. S. Shames, JD Minna, AF Gazdar: DNA methylation in health, disease, and cancer . In: Curr. Mol. Med. Band 7 , no. 1 , February 2007, p. 85-102 , PMID 17311535 .
- ↑ S. Beck, VK Rakyan: The methylome: approaches for global DNA methylation profiling . In: Trends Genet . tape 24 , no. 5 , May 2008, pp. 231-237 , doi : 10.1016 / j.tig.2008.01.006 , PMID 18325624 .
- ↑ L. Shen, RA Waterland: Methods of DNA methylation analysis . In: Curr Opin Clin Nutr Metab Care . tape 10 , no. 5 , September 2007, pp. 576-581 , doi : 10.1097 / MCO.0b013e3282bf6f43 , PMID 17693740 .
- ↑ a b B. A. Braaten, X. Nou, LS Kaltenbach, DA Low: Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. In: Cell. Volume 76, Number 3, February 1994, pp. 577-588. PMID 7906204 .
- ↑ a b c d e f Matthew J. Blow, Tyson A. Clark, Chris G. Daum, Adam M. Deutschbauer, Alexey Fomenkov, Roxanne Fries, Jeff Froula, Dongwan D. Kang, Rex R. Malmstrom, Richard D. Morgan , Janos Posfai, Kanwar Singh, Axel Visel, Kelly Wetmore, Zhiying Zhao, Edward M. Rubin, Jonas Korlach, Len A. Pennacchio, Richard J. Roberts: The Epigenomic Landscape of Prokaryotes. In: PLoS Genet. 12 (2), Feb 2016, p. E1005854. doi: 10.1371 / journal.pgen.1005854 . PMID 26870957 . PMC 4752239 (free full text).
- ↑ MM Pooggin: How can plant DNA viruses evade siRNA-directed DNA methylation and silencing? In: International journal of molecular sciences. Volume 14, number 8, July 2013, pp. 15233-15259, doi : 10.3390 / ijms140815233 , PMID 23887650 , PMC 3759858 (free full text) (review).
- ↑ S. Mukherjee, VC Vipat, AK Chakrabarti: Infection with influenza A viruses causes changes in promoter DNA methylation of inflammatory genes. In: Influenza and other respiratory viruses. Volume 7, number 6, November 2013, pp. 979-986, doi : 10.1111 / irv.12127 , PMID 23758996 , PMC 4634256 (free full text).
- ^ JE Shin, C. Lin, HN Lim: Horizontal transfer of DNA methylation patterns into bacterial chromosomes. In: Nucleic acids research. Volume 44, number 9, 05 2016, pp. 4460–4471, doi : 10.1093 / nar / gkw230 , PMID 27084942 , PMC 4872104 (free full text).
- ^ DB Dunn, JD Smith: The occurrence of 6-methylaminopurine in deoxyribonucleic acids. In: Biochem J. 68 (4), Apr 1958, pp. 627-636. PMID 13522672 . PMC 1200409 (free full text).
- ↑ BF Vanyushin, SG Tkacheva, AN Belozersky: Rare bases in animal DNA. In: Nature. 225, 1970, pp. 948-949. PMID 4391887 .
- ↑ a b Melanie Ehrlich, Miguel A. Gama-Sosa, Laura H. Carreira, Lars G. Ljungdahl, Kenneth C. Kuo, Charles W. Gehrke: DNA methylation in thermophilic bacteria: N6-methylcytosine, 5-methylcytosine, and N6- methyladenine. In: Nucleic Acids Research. 13, 1985, p. 1399. PMID 4000939 . PMC 341080 (free full text).
- ↑ S. Hattman, C. Kenny, L. Berger, K. Pratt: Comparative study of DNA methylation in three unicellular eucaryotes. In: J Bacteriol. 135 (3), Sep 1978, pp. 1156-1157. PMID 99431 . PMC 222496 (free full text).
- ↑ D. Ratel, J.-L. Ravanat, M.-P. Charles, N. Platet, L. Breuillaud, J. Lunardi, F. Berger, D. Wion: Undetectable levels of N6-methyl adenine in mouse DNA: Cloning and analysis of PRED28, a gene coding for a putative mammalian DNA adenine methyltransferase. In: FEBS Lett. 580, 2006, pp. 3179-3184. PMID 16684535 . doi: 10.1016 / j.febslet.2006.04.074 .
- ↑ Eric Lieberman Greer, Mario Andres Blanco, Lei Gu, Erdem Sendinc, Jianzhao Liu: DNA Methylation on N6-Adenine in C. elegans . In: Cell . tape 161 , no. 4 , May 7, 2015, ISSN 1097-4172 , p. 868-878 , doi : 10.1016 / j.cell.2015.04.005 , PMID 25936839 , PMC 4427530 (free full text).
- ↑ Guoqiang Zhang, Hua Huang, Di Liu, Ying Cheng, Xiaoling Liu: N6-methyladenine DNA modification in Drosophila . In: Cell . tape 161 , no. 4 , May 7, 2015, ISSN 1097-4172 , p. 893-906 , doi : 10.1016 / j.cell.2015.04.018 , PMID 25936838 .
- ↑ F. Capuano, M. Mulleder, R. Kok, HJ Blom, M. Ralser: Cytosine DNA Methylation Is Found in Drosophila melanogaster but Absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Other Yeast Species. In: Analytical Chemistry. 86, 2014, pp. 3697-3702. doi: 10.1021 / ac500447w . PMID 24640988 . PMC PMC4006885 (free full text).
- ↑ a b Guan-Zheng Luo, Mario Andres Blanco, Eric Lieberman Greer, Chuan He, Yang Shi: DNA N6-methyladenine: a new epigenetic mark in eukaryotes? In: Nature Reviews Molecular Cell Biology. 16, 2015, p. 705, doi: 10.1038 / nrm4076 . PMID 26507168 . PMC 4763336 (free full text).
- ^ J. Casadesus, D. Low: Epigenetic gene regulation in the bacterial world. In: Microbiology and molecular biology reviews: MMBR. Volume 70, Number 3, September 2006, pp. 830-856, doi: 10.1128 / MMBR.00016-06 . PMID 16959970 , PMC 1594586 (free full text).
- ↑ D. Wion, J. Casadesus: N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. In: Nature reviews. Microbiology. Volume 4, Number 3, March 2006, pp. 183-192, doi: 10.1038 / nrmicro1350 . PMID 16489347 , PMC 2755769 (free full text) (review).
- ^ R. Kumar, DN Rao: Role of DNA methyltransferases in epigenetic regulation in bacteria. In: Epigenetics: Development and Disease. (= Subcellular Biochemistry. 61). 2013, pp. 81-102. doi : 10.1007 / 978-94-007-4525-4_4 . PMID 23150247 .
- ↑ J. Casadesus, DA Low: Programmed heterogeneity: epigenetic mechanisms in bacteria. In: J Biol Chem. 288 (20), May 17, 2013, pp. 13929-13935. doi: 10.1074 / jbc.R113.472274 . PMID 23592777 . PMC 3656251 (free full text).
- ↑ BM Forde, MD Phan, JA Gawthorne, MM Ashcroft, M. Stanton-Cook, S. Sarkar, KM Peters, KG Chan, TM Chong, WF Yin, M. Upton, MA Schembri, SA Beatson: Lineage-Specific Methyltransferases Define the Methylome of the Globally Disseminated Escherichia coli ST131 Clone. In: mBio. Volume 6, number 6, November 2015, pp. E01602 – e01615, doi: 10.1128 / mBio.01602-15 . PMID 26578678 , PMC 4659465 (free full text).
- ↑ Robson Ee, Yan-Lue Lim, Wai-Fong Yin, Wah-Seng See-Too, Richard J. Roberts, Kok-Gan Chan: Novel Methyltransferase Recognition Motif Identified in Chania multitudinisentens RB-25T gen. Nov., Sp. nov. In: Frontiers in Microbiology. 7, 2016, doi: 10.3389 / fmicb.2016.01362 .
- ↑ F. Barras, MG Marinus: The great GATC: DNA methylation in E. coli. In: Trends Genet. Vol. 5, No. 5, 1989, pp. 139-143. PMID 2667217 .
- ^ MG Marinus, J. Casadesus: Roles of DNA adenine methylation in host-pathogen interactions: mismatch repair, transcriptional regulation, and more. In: FEMS Microbiol Rev . Volume 33, No. 3, 2009, pp. 488-503. PMID 19175412 , doi: 10.1111 / j.1574-6976.2008.00159.x .
- ↑ a b c J. Casadesús, DA Low: Programmed heterogeneity: epigenetic mechanisms in bacteria. In: The Journal of Biological Chemistry . Volume 288, number 20, May 2013, pp. 13929-13935, doi : 10.1074 / jbc.R113.472274 , PMID 23592777 , PMC 3656251 (free full text) (review).
- ↑ J. Errington: Regulation of endospore formation in Bacillus subtilis. In: Nature reviews . Microbiology. Volume 1, Number 2, November 2003, pp. 117-126, doi : 10.1038 / nrmicro750 , PMID 15035041 (review).
- ↑ KE Gibson, H. Kobayashi, GC Walker: Molecular determinants of a symbiotic chronic infection. In: Annual Review of Genetics . Volume 42, 2008, pp. 413-441, doi : 10.1146 / annurev.genet.42.110807.091427 , PMID 18983260 , PMC 2770587 (free full text) (review).
- ↑ CL Kirkpatrick, PH Viollier: Decoding Caulobacter development. In: FEMS Microbiology Reviews . Volume 36, Number 1, January 2012, pp. 193-205, doi : 10.1111 / j.1574-6976.2011.00309.x , PMID 22091823 (review).
- ^ D. Kaiser: Myxococcus-from single-cell polarity to complex multicellular patterns. In: Annual Review of Genetics . Volume 42, 2008, pp. 109-130, doi : 10.1146 / annurev.genet.42.110807.091615 , PMID 18605899 (review).
- ↑ E. Flores, A. Herrero: Compartmentalized function through cell differentiation in filamentous cyanobacteria. In: Nature Reviews . Microbiology. Volume 8, Number 1, January 2010, pp. 39-50, doi : 10.1038 / nrmicro2242 , PMID 19966815 (review).
- ^ PS Stewart, MJ Franklin: Physiological heterogeneity in biofilms. In: Nature Reviews Microbiology . Volume 6, Number 3, March 2008, pp. 199-210, doi : 10.1038 / nrmicro1838 , PMID 18264116 (review).
- ^ Y. Chai, F. Chu, R. Kolter, R. Losick: Bistability and biofilm formation in Bacillus subtilis. In: Molecular Microbiology . Volume 67, number 2, January 2008, pp. 254-263, doi : 10.1111 / j.1365-2958.2007.06040.x , PMID 18047568 , PMC 2430929 (free full text).
- ^ CH Waddington: The Strategy of the Genes. Published by George Allen and Unwin, London.
- ↑ a b L. B. Blyn, BA Braaten, DA Low: Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. In: The EMBO Journal . Volume 9, Number 12, December 1990, pp. 4045-4054, PMID 2147413 , PMC 552177 (free full text).
- ↑ a b M. X. Wang, GM Church: A whole genome approach to in vivo DNA-protein interactions in E. coli. In: Nature . Volume 360, Number 6404, December 1992, pp. 606-610, doi : 10.1038 / 360606a0 , PMID 1334233 .
- ↑ M. van der Woude, WB Hale, DA Low: Formation of DNA methylation patterns: nonmethylated GATC sequences in gut and pap operons. In: Journal of Bacteriology . Volume 180, Number 22, November 1998, pp. 5913-5920, PMID 9811649 , PMC 107665 (free full text).
- ↑ DE Waldron, P. Owen, CJ Dorman: Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. In: Molecular Microbiology . Volume 44, Number 2, April 2002, pp. 509-520, PMID 11972787 .
- ↑ S. Ringquist, CL Smith: The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. In: Proceedings of the National Academy of Sciences . Volume 89, Number 10, May 1992, pp. 4539-4543, PMID 1584789 , PMC 49118 (free full text).
- ↑ Olivier Mathieu, Georges Picard, Sylvette Tourmente: Methylation of a euchromatin-heterochromatin transition region in Arabidopsis thaliana chromosome 5 left arm . In: Chromosome Research: An International Journal on the Molecular, Supramolecular and Evolutionary Aspects of Chromosome Biology . tape 10 , no. 6 , 2002, ISSN 0967-3849 , p. 455-466 , PMID 12489828 .
- ↑ M. Gardiner-Garden, M. Frommer: CpG islands in vertebrate genomes. In: Journal of molecular biology. Volume 196, Number 2, July 1987, pp. 261-282. PMID 3656447 .
- ↑ GD Ginder, DC Williams: Readers of DNA methylation, the MBD family as potential therapeutic targets. In: Pharmacology & therapeutics. [electronic publication before printing] November 2017, doi: 10.1016 / j.pharmthera.2017.11.002 . PMID 29128342 (Review).
- ↑ PD Fransquet, P. Lacaze, R. Saffery, J. McNeil, R. Woods, J. Ryan: Blood DNA methylation as a potential biomarker of dementia: A systematic review. In: Alzheimer's & dementia: the journal of the Alzheimer's Association. [electronic publication before printing] November 2017, doi: 10.1016 / j.jalz.2017.10.002 . PMID 29127806 (Review).
- ↑ a b F. Capuano, M. Mülleder, R. Kok, HJ Blom, M. Ralser: Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. In: Analytical chemistry. Volume 86, Number 8, April 2014, pp. 3697-3702, doi: 10.1021 / ac500447w . PMID 24640988 , PMC 4006885 (free full text).
- ^ X. Cao, SE Jacobsen: Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. In: Proceedings of the National Academy of Sciences . Volume 99 Suppl 4, December 2002, pp. 16491-16498, doi: 10.1073 / pnas.162371599 . PMID 12151602 , PMC 139913 (free full text).
- ↑ a b W. essay, MF Mette, J. van der Winden, AJ Matzke, M. Matzke: RNA-directed DNA methylation in Arabidopsis. In: Proceedings of the National Academy of Sciences . Volume 99 Suppl 4, December 2002, pp. 16499-16506, doi: 10.1073 / pnas.162371499 . PMID 12169664 , PMC 139914 (free full text).
- ^ Y. Wang, M. Jorda, PL Jones, R. Maleszka, X. Ling, HM Robertson, CA Mizzen, MA Peinado, GE Robinson: Functional CpG methylation system in a social insect. In: Science. Volume 314, number 5799, October 2006, pp. 645-647, doi: 10.1126 / science.1135213 . PMID 17068262 .
- ^ Y. Wang, H. Li-Byarlay: Physiological and Molecular Mechanisms of Nutrition in Honey Bees. In: Advances in Insect Physiology. Volume 49, Chapter 2, August 2015, pp. 25-85, doi: 10.1016 / bs.aiip.2015.06.002 .
- ↑ H. Li-Byarlay, Y. Li, H. Stroud, S. Feng, TC Newman, M. Kaneda, KK Hou, KC Worley, CG Elsik, SA Wickline, SE Jacobsen, J. Ma, GE Robinson: RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. In: Proceedings of the National Academy of Sciences . Volume 110, Number 31, July 2013, pp. 12750-12755, doi: 10.1073 / pnas.1310735110 . PMID 23852726 , PMC 3732956 (free full text).
- ^ SS Smith, CA Thomas: The two-dimensional restriction analysis of Drosophila DNAs: males and females. In: Genes. Volume 13, Number 4, May 1981, pp. 395-408. PMID 6266924 .
- ↑ F. Lyko, BH Ramsahoye, R. Jaenisch: DNA methylation in Drosophila melanogaster. In: Nature. Volume 408, number 6812, November 2000, pp. 538-540, doi: 10.1038 / 35046205 . PMID 11117732 .
- Jump up ↑ S. Takayama, J. Dhahbi, A. Roberts, G. Mao, SJ Heo, L. Pachter, DI Martin, D. Boffelli: Genome methylation in D. melanogaster is found at specific short motifs and is independent of DNMT2 activity. In: Genome research. Volume 24, number 5, May 2014, pp. 821–830, doi: 10.1101 / gr.162412.113 . PMID 24558263 , PMC 4009611 (free full text).
- ↑ G. Zhang, H. Huang, D. Liu, Y. Cheng, X. Liu, W. Zhang, R. Yin, D. Zhang, P. Zhang, J. Liu, C. Li, B. Liu, Y Luo, Y. Zhu, N. Zhang, S. He, C. He, H. Wang, D. Chen: N6-methyladenine DNA modification in Drosophila. In: Cell. Volume 161, number 4, May 2015, pp. 893-906, doi: 10.1016 / j.cell.2015.04.018 . PMID 25936838 .
- ↑ F. Antequera, M. Tamame, JR Villanueva, T. Santos: DNA methylation in the fungi. In: The Journal of biological chemistry. Volume 259, Number 13, July 1984, pp. 8033-8036. PMID 6330093 .
- ↑ Thomas Binz, Nisha D'Mello, Paul A. Horgen: A Comparison of DNA Methylation Levels in Selected Isolates of Higher Fungi. In: Mycologia. 90, 1998, p. 785, doi: 10.2307 / 3761319 .
- ↑ SY Liu, JQ Lin, HL Wu, CC Wang, SJ Huang, YF Luo, JH Sun, JX Zhou, SJ Yan, JG He, J. Wang, ZM He: Bisulfite sequencing reveals that Aspergillus flavus holds a hollow in DNA methylation . In: PloS one. Volume 7, number 1, 2012, p. E30349, doi: 10.1371 / journal.pone.0030349 . PMID 22276181 , PMC 3262820 (free full text).
- ↑ EU Selker, NA Tountas, SH Cross, BS Margolin, JG Murphy, AP Bird, M. Freitag: The methylated component of the Neurospora crassa genome. In: Nature. Volume 422, number 6934, April 2003, pp. 893-897, doi: 10.1038 / nature01564 . PMID 12712205 .
- ^ SS Smith, DI Ratner: Lack of 5-methylcytosine in Dictyostelium discoideum DNA. In: The Biochemical journal. Volume 277 (Pt 1), July 1991, pp. 273-275. PMID 1713034 , PMC 1151219 (free full text).
- ↑ JL Steenwyk, J. St-Denis, J. Dresch, D. Larochelle, RA Drewell: Whole genome bisulfite sequencing reveals a sparse, but robust pattern of DNA methylation in the Dictyostelium discoideum genome. In: bioRxiv. 2017, 166033. doi: 10.1101 / 166033 .
- ↑ JG Reilly, R. Braun, CA Thomas: Methjylation in Physarum DNA. In: FEBS letters. Volume 116, Number 2, July 1980, pp. 181-184. PMID 6250882 .
- ^ HH Evans, TE Evans: Methylation of the deoxyribonucleic acid of Physarum polycephalum at various periods during the mitotic cycle. In: The Journal of biological chemistry. Volume 245, Number 23, December 1970, pp. 6436-6441. PMID 5530731 .
- ↑ a b A. Jeltsch: Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. In: ChemBioChem Volume 3, No. 4, 2002, pp. 274-293. PMID 11933228 .
- ↑ A. Jeltsch: Molecular enzymology of mammalian DNA methyltransferases. In: Curr Top Microbiol Immunol. Volume 301, 2006, pp. 203-225. PMID 16570849 .
- ^ S. Seisenberger, JR Peat, TA Hore, F. Santos, W. Dean, W. Reik: Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. In: Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Volume 368, number 1609, January 2013, p. 20110330, doi: 10.1098 / rstb.2011.0330 . PMID 23166394 . PMC 3539359 (free full text).
- ↑ MJ Snider, L. Reinhardt, R. Wolfenden, WW Cleland: 15N kinetic isotope effects on uncatalyzed and enzymatic deamination of cytidine. In: Biochemistry. Volume 41, Number 1, January 2002, pp. 415-421, PMID 11772041 .
- ↑ MJ Snider, R. Wolfenden: Site-bound water and the shortcomings of a less than perfect transition state analogue. In: Biochemistry. Volume 40, Number 38, September 2001, pp. 11364-11371, PMID 11560484 .
- ↑ Vincent Caval, Rodolphe Suspène, Jean-Pierre Vartanian, Simon Wain-Hobson: Orthologous Mammalian APOBEC3A Cytidine Deaminases Hypermutate Nuclear DNA. In: Molecular Biology and Evolution. 31, 2014, p. 330, doi : 10.1093 / molbev / mst195 .
- ↑ a b N. Schormann, R. Ricciardi, D. Chattopadhyay: Uracil-DNA glycosylases-structural and functional perspectives on an essential family of DNA repair enzymes. In: Protein science: a publication of the Protein Society. Volume 23, number 12, December 2014, pp. 1667–1685, doi : 10.1002 / pro.2554 , PMID 25252105 , PMC 4253808 (free full text) (review).
- ↑ N. Cervoni, S. Bhattacharya, M. Szyf: DNA demethylase is a processive enzyme . In: The Journal of Biological Chemistry . tape 274 , no. 13 , March 26, 1999, ISSN 0021-9258 , p. 8363-8366 , doi : 10.1074 / jbc.274.13.8363 , PMID 10085064 .
- ↑ H. Cedar, Y. Bergman: Programming of DNA methylation patterns. In: Annual review of biochemistry. Volume 81, 2012, pp. 97-117, doi : 10.1146 / annurev-biochem-052610-091920 , PMID 22404632 (review).
- ↑ C. Beard, E. Li, R. Jaenisch: Loss of methylation activates X is in somatic but not in embryonic cells. In: Genes & development. Volume 9, Number 19, October 1995, pp. 2325-2334, PMID 7557385 .
- ↑ E. Li, C. Beard, R. Jaenisch: Role for DNA methylation in genomic imprinting. In: Nature. Volume 366, Number 6453, November 1993, pp. 362-365, doi : 10.1038 / 366362a0 , PMID 8247133 .
- ↑ Entrez Gene: XIST X (inactive) -specific transcript
- ↑ J. Borgel, S. Guibert, Y. Li, H. Chiba, D. Schübeler, H. Sasaki, T. Forné, M. Weber: Targets and dynamics of promoter DNA methylation during early mouse development. In: Nature Genetics. 42, 2010, p. 1093, doi : 10.1038 / ng.708 .
- ^ S. Seisenberger, JR Peat, TA Hore, F. Santos, W. Dean, W. Reik: Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. In: Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Volume 368, number 1609, January 2013, p. 20110330, doi : 10.1098 / rstb.2011.0330 , PMID 23166394 , PMC 3539359 (free full text) (review).
- ↑ MJ Ziller, F. Müller, J. Liao, Y. Zhang, H. Gu, C. Bock, P. Boyle, CB Epstein, BE Bernstein, T. Lengauer, A. Gnirke, A. Meissner: Genomic distribution and inter -sample variation of non-CpG methylation across human cell types. In: PLoS genetics. Volume 7, number 12, December 2011, p. E1002389, doi : 10.1371 / journal.pgen.1002389 , PMID 22174693 , PMC 3234221 (free full text).
- ↑ a b M. Fasolino, Z. Zhou: The Crucial Role of DNA Methylation and MeCP2 in Neuronal Function. In: Genes. Volume 8, number 5, May 2017, p., Doi : 10.3390 / genes8050141 , PMID 28505093 , PMC 5448015 (free full text) (review).
- ↑ a b c C. G. Duke, AJ Kennedy, CF Gavin, JJ Day, JD Sweatt: Experience-dependent epigenomic reorganization in the hippocampus. In: Learning & memory. Volume 24, number 7, 07 2017, pp. 278–288, doi : 10.1101 / lm.045112.117 , PMID 28620075 , PMC 5473107 (free full text).
- ^ R. Halder, M. Hennion, RO Vidal, O. Shomroni, RU Rahman, A. Rajput, TP Centeno, F. van Bebber, V. Capece, JC Garcia Vizcaino, AL Schuetz, S. Burkhardt, E. Benito, M. Navarro Sala, SB Javan, C. Haass, B. Schmid, A. Fischer, S. Bonn: DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. In: Nature Neuroscience . Volume 19, Number 1, January 2016, pp. 102-110, doi : 10.1038 / nn.4194 , PMID 26656643 .
- ↑ H. Heyn, N. Li et al .: Distinct DNA methylomes of newborns and centenarians. In: Proceedings of the National Academy of Sciences . Volume 109, Number 26, June 2012, pp. 10522-10527. doi: 10.1073 / pnas.1120658109 . PMID 22689993 .
- ↑ AA Johnson, K. Akman, SR Calimport, D. Wuttke, A. Stolzing, JP de Magalhães: The role of DNA methylation in aging, rejuvenation, and age-related disease. In: Rejuvenation research. Volume 15, number 5, October 2012, pp. 483-494, doi: 10.1089 / rej.2012.1324 . PMID 23098078 . PMC 3482848 (free full text).
- ^ JA Hackett, MA Surani: DNA methylation dynamics during the mammalian life cycle. In: Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Volume 368, number 1609, January 2013, p. 20110328, doi: 10.1098 / rstb.2011.0328 . PMID 23166392 . PMC 3539357 (free full text).
- ↑ M. Berdasco, M. Esteller: DNA methylation in stem cell renewal and multipotency. In: Stem cell research & therapy. Volume 2, number 5, 2011, p. 42, doi: 10.1186 / scrt83 . PMID 22041459 . PMC 3308039 (free full text).
- ↑ Keh-Yang Wang, Chun-Chang Chen, Shih-Feng Tsai, Che-Kun James Shen: Epigenetic Enhancement of the Post-replicative DNA Mismatch Repair of Mammalian Genomes by a Hemi-mCpG-Np95-Dnmt1 Axis. In: Scientific Reports. 6, 2016, p. 37490, doi: 10.1038 / srep37490 . PMC 5122852 (free full text). PMID 27886214 .
- ↑ E. Daura-Oller, M. Cabre, MA Montero, JL Paternain, A. Romeu: Specific gene hypomethylation and cancer: new insights into coding region feature trends . In: Bioinformation . tape 3 , no. 8 , 2009, p. 340-343 , PMID 19707296 , PMC 2720671 (free full text).
- ↑ RL Momparler, V. Bovenzi: DNA methylation and cancer. In: J. Cell Physiol. Volume 183, No. 2, 2000, pp. 145-154. PMID 10737890 .
- ↑ PV Wijermans include: An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2'-deoxycytidine (decitabine) in 177 patients. In: Ann. Hematol. Volume 84, No. 1, 2005, pp. 9-17. PMID 16211386 , doi: 10.1007 / s00277-005-0012-1 .
- ↑ Dacogen clearance by the FDA .
- ↑ Gerda Egger, Gangning Liang, Ana Aparicio, Peter A. Jones: Epigenetics in human disease and prospects for epigenetic therapy . In: Nature . tape 429 , no. 6990 , May 27, 2004, ISSN 1476-4687 , p. 457-463 , doi : 10.1038 / nature02625 , PMID 15164071 .
- ^ Adrian Bird: Perceptions of epigenetics . In: Nature . tape 447 , no. 7143 , May 24, 2007, ISSN 1476-4687 , p. 396-398 , doi : 10.1038 / nature05913 , PMID 17522671 .
- ^ CH Waddington: Towards a Theoretical Biology . In: Nature . tape 218 , no. 5141 , May 1968, ISSN 0028-0836 , p. 525-527 , doi : 10.1038 / 218525a0 .
- ↑ CH Waddington: The epigenotype. 1942 . In: International Journal of Epidemiology . tape 41 , no. 1 , February 2012, ISSN 1464-3685 , p. 10-13 , doi : 10.1093 / ije / dyr184 , PMID 22186258 .