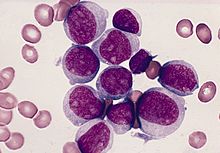
Acute myeloid leukemia
| Classification according to ICD-10 | |
|---|---|
| C92.0 | Acute myeloid leukemia |
| ICD-10 online (WHO version 2019) | |
| Classification according to ICD-O-3 | |
|---|---|
| 9861/3 | Acute myeloid leukemia nos |
| ICD-O-3 first revision online | |
The acute myeloid leukemia ( AML ) is a malignant (malignant) disease of the hematopoietic system, namely the engraftment , so that part of the hematopoietic system, which for the formation of granulocytes , monocytes , erythrocytes and megakaryocytes is responsible. It leads to a massive increase in immature precursors of myelopoiesis in the bone marrow and, in the majority of cases, also in the blood ( leukocytosis ).
history
The term "leukemia" was coined in 1845 by Rudolf Virchow , who at that time described the clinical picture of chronic myeloid leukemia . The development of staining methods for blood smears in 1891 by Paul Ehrlich led to new knowledge about the morphology of acute leukemia and subsequently made it possible to differentiate between myeloid and lymphatic leukemia (Naegeli 1900).
Epidemiology
AML is a rare disease with an incidence of about three new cases / 100,000 per year. Around 3,600 new cases occur in Germany each year. It is predominantly a disease of older age; the median age at diagnosis is 63 years. AML accounts for about 80% of all acute leukaemias in adults. Men are affected slightly more often than women (ratio 1.4: 1). In childhood, only 15 to 20% of patients with acute leukemia have AML. However, the rare acute leukemia in newborns is usually AML.
Causes and origins
Acquired AML
Known risk factors for the development of AML are exposure to high doses of ionizing radiation (e.g. after the atomic bomb explosions in Hiroshima and Nagasaki ) and long-term chronic exposure to benzene . Even after using certain cytotoxic drugs , such as alkylating agents and etoposide , AML can develop after a latency period of several years. Smoking also plays a role in the development of AML. In many cases, however, the cause remains unclear.
Genetic factors resp. causes
The mechanisms that lead to the development of AML are the subject of current research. Today it is assumed that at the beginning of the development of leukemia there are genetic changes in a single hematopoietic precursor cell. These changes lead to a clonal proliferation of immature cells that have lost the ability to mature. Numerous chromosomal aberrations known in AML (see below: Cytogenetics section ) often involve genes that play an important role in normal cell regulation . By translocations arise partly new fusion genes that are involved in leukemia development, eg. B. PML / RARα at t (15; 17), AML1 / ETO at t (8; 21).
AML is more common in some genetic diseases such as: B. Down syndrome or Fanconi anemia .
Clinic and course
The leukemic cells spread to the bone marrow and blood and can also infiltrate lymph nodes , spleen and other organs, and in rare cases the central nervous system . The immediate result is a suppression of normal blood formation ( hematopoiesis ). There is a shortage of erythrocytes, blood platelets and functional mature granulocytes.
The symptoms of AML are predominantly due to bone marrow insufficiency. It is often an acute clinical picture, typical symptoms are:
- General weakness, malaise, paleness
- Night sweats
- Signs of a bleeding disorder with petechiae , hematoma, or bleeding from the mucous membranes or gums
- Infections of different localization such as B. Pneumonia ("pneumonia"), tonsillitis (" tonsillitis ") or unclear fever
- Inflammation of the lining of the mouth, oral thrush
- Rarely moderate splenomegaly and / or lymphadenopathy, occasionally swelling of the gums ( gingival hyperplasia ), these changes are mainly in the monocytic leukemia ago
Other frequent findings are:
- In most cases leukocytosis , sometimes normal or even low white blood cell count
- Occurrence of blasts in the differential blood count
- Anemia and thrombopenia
- Increase in LDH , ESR and uric acid
- Coagulation disorders (predominantly in promyelocyte leukemia or monocytic leukemia)
If left untreated, the disease progresses quickly and leads to death after a few weeks, mostly due to uncontrollable infection or bleeding.
Diagnostics and classification
The suspected diagnosis of acute leukemia is based on the clinical symptoms and the blood count, including the differential blood count . The diagnosis is confirmed by examining the bone marrow (see bone marrow puncture ). The differentiation between AML and acute lymphoblastic leukemia (ALL) is crucial for treatment and prognosis .
Modern diagnostics are based on a combination of morphological and cytochemical findings, supplemented by immunophenotyping and cytogenetics and, if necessary, molecular diagnostics. In the future, the analysis of gene expression profiles using microarray technology will probably gain increasing importance.
Diagnosing AML requires:
- evidence of a proportion of immature blasts of at least 30% ( FAB classification - French-American-British) or 20% ( WHO classification) in the bone marrow.
- the assignment of the blasts to the myeloid series by cytochemical examination and / or immunophenotype.
- the further assignment to an AML subtype according to the FAB classification or WHO classification.
Morphology and Cytochemistry
The basis of diagnosis is the microscopic examination of bone marrow smears. Characteristic features such as B. the detection of Auer rods enable the assignment of the blasts to the myeloid series. In Auer rods is delicate, rod-shaped granules or large, oval to elliptiforme inclusions (Auer bodies) in the cytoplasm of immature leukemic cells. If necessary with the help of additional cytochemical examinations (peroxidase, esterase, PAS reaction ), in the majority of cases it is possible to differentiate AML from ALL and to classify it according to the FAB classification.
| FAB subtype | designation | Cytogenetic aberrations |
|---|---|---|
| M0 | Acute myeloid leukemia with minimal differentiation | - |
| M1 | Acute myeloid leukemia without maturation | - |
| M2 | Acute myeloid leukemia with maturation | t (8; 21) |
| M3 | Acute Promyelocyte Leukemia (APL) | t (15; 17) |
| M3v | Acute promyelocyte leukemia, microgranular form | t (15; 17) |
| M4 | Acute myelomonocytic leukemia | - |
| M4Eo | Acute myelomonocytic leukemia with eosinophilia | inv (16) |
| M5a | Acute monoblastic leukemia | - |
| M5b | Acute monocyte leukemia | - |
| M6 | Acute erythroleukemia | - |
| M7 | Acute megakaryoblastic leukemia | - |
A further development of the FAB classification is the WHO classification, which includes frequent chromosomal aberrations in AML. The promyelocytic leukemia (FAB M3 or m3v) has clinical, biological and therapeutic special features and will be discussed in a separate article.
Immunophenotyping
The immunophenotyping has in the AML primarily as corroboration, but can deliver in doubt, important additional information. With the help of labeled monoclonal antibodies , the expression of membrane-bound surface molecules on the leukemia cells is examined. In most cases, these are differentiation antigens which are also expressed in the course of normal hematopoiesis and are summarized in the so-called CD nomenclature . The most important antigens for characterizing an AML are summarized in the following table.
| antigen | |
|---|---|
| Pan-myeloid | CD13, CD33, CD65s |
| Granulocytic, monocytic | CD15, CD14, CD64 |
| Progenitor cells | CD34, CD117, CD7 |
Cytogenetics
Most patients with AML have acquired numerical and structural chromosomal aberrations in the leukemia cells. Some of these aberrations are closely related to or define a particular morphological and clinical subtype. In recent years the diagnostic, prognostic and therapeutic significance of the cytogenetic changes in AML has become increasingly clear. Common chromosomal aberrations are:
| Aberration | Special features |
|---|---|
| +8 | Most common numerical chromosomal aberration in AML |
| t (8; 21) | Most common structural chromosomal aberration in AML |
| t (15; 17) | Specific to promyelocytic leukemia |
| t (9; 22) | so-called " Philadelphia chromosome " |
| inv (16) | M4Eo |
Therapy and prognosis
Prognostic factors
A de novo AML is differentiated from a secondary AML. Both forms differ in terms of the biology of the disease and the therapeutic response. A secondary AML is used when the disease is associated with a haematological disease, e.g. B. myelodysplastic syndrome (MDS), or has been exposed to cytostatics or radiation. Secondary AML shows some cytogenetic changes that are unfavorable in terms of prognosis and is less responsive to therapy.
A number of prognostic factors can be used to estimate the response to therapy. However, these factors are not independent of one another, e.g. B. Complex cytogenetic aberrations are more common in secondary AML.
Unfavorable prognosis a .:
- Old age and / or poor general condition at diagnosis
- High white blood cell count
- Previous haematological disease (e.g. MDS, blast flare in CML ), secondary AML
- FAB M0, FAB M6, FAB M7
- Unfavorable cytogenetics ( trisomy 8 , aberrations of chromosome 5 or 7, t (6; 9), t (9; 22), 11q23 aberrations, complex cytogenetic aberrations)
- Secondary leukemia
Favorable prognostic factors are:
- Promyelocyte Leukemia (FAB M3)
- Favorable cytogenetics: t (15; 17), inv (16), t (8; 21), normal karyotype
Therapy implementation
The basis of AML therapy is intensive chemotherapy . The aim of the so-called induction therapy is to achieve a complete remission, ie to eliminate all symptoms of the disease with normalization of the blood count and elimination of the pathological cell population in the bone marrow (blasts <5%). The treatment consists of several days of therapy that are repeated several times (two to three times). With these, the patient comes into so-called "isolation"; the number of leukocytes is so low that every infection can be fatal under certain circumstances, which requires an absolute mask to be worn when dealing with patients. Important drugs are cytarabine and the anthracyclines daunorubicin and idarubicin . If necessary, thioguanine is also given. Once a complete remission has been achieved (usually after 1–2 therapy blocks), post-remission therapy follows. It includes consolidation therapy (high-dose cytarabine) and, in acute promyelocytic leukemia, maintenance therapy ( all-trans retinol , 6- mercaptopurine and methotrexate ). Allogeneic or autologous stem cell transplantation can also be considered as further therapeutic methods .
Azacitidine is approved for the treatment of acute myeloid leukemia with 20-30% blasts and multiline dysplasia according to the WHO classification.
The significance of new therapeutic approaches, such as the immunotoxin gemtuzumab-ozogamicin , which is only approved in the United States, is still unclear.
In the accompanying therapy, the replacement of blood components ( erythrocytes , thrombocytes ), possibly the use of growth factors for granulocytes (e.g. G-CSF) and the fight against infections with antibiotics against bacterial infections and antimycotics against fungal infections play an essential role.
The chemotherapy can also be supported by the administration of all-trans retinoic acid (ATRA). In 2018 Küley-Bagheri et al. produced a Cochrane review of randomized controlled phase II and III studies to assess the advantages and disadvantages of ATRA as an adjunct to chemotherapy in patients with AML.
Chemotherapy and stem cell transplantation result in side effects such as bleeding or transplant-versus-host reactions (only with stem cell transplantation). Cochrane Haematology has therefore prepared some reviews to evaluate how these side effects can be treated or avoided. Estcourt et al. conducted Cochrane reviews of randomized controlled trials in 2012 and 2015 to find out which use of platelet transfusions is most effective in preventing bleeding in patients with haematological disorders when receiving chemotherapy or a stem cell transplant. In 2019, Fisher et al. created a Cochrane review of randomized controlled trials to measure the safety and efficacy of mesenchymal stromal cells (MSC) in patients with a graft-versus-host reaction (GvHD).
Therapy results
With induction therapy, depending on the patient selection, it is possible to achieve a complete remission in around 70% of patients with AML.
The treatment results in older patients (> 60 years) are significantly worse, the rate of complete remissions is between 30% and 60%. The cause is not only concomitant diseases in old age that lead to complications, but also the increased occurrence of prognostically unfavorable factors such as unfavorable cytogenetics or secondary leukemia after previous myelodysplastic syndrome . Unfortunately, a relapse is to be expected in the majority of patients, around 15% -25% achieve long-term remissions after conventional chemotherapy and can be regarded as cured. The results after allogeneic stem cell transplantation are more favorable. In a nationwide clinical study with over 220 patients, which was carried out between 2003 and 2009, the use of low-dose decitabine for the treatment of elderly patients was examined.
Much better therapy results are obtained for promyelocytic leukemia.
Supportive therapy
The therapy of the disease can be supported by supportive measures. This can include physical activity. As part of the Cochrane-Wikipedia partnership , current research results are inserted here. In 2019, Knips et al. conducted a Cochrane review of randomized controlled trials to re-evaluate the safety, efficacy and feasibility of exercise in addition to standard care in adult patients with malignant haematological diseases. The exact inclusion and exclusion criteria and further details can be found in the original Cochrane review. The study participants had disease stages I to IV. Since physical activity was only an additional treatment, the patients also received chemotherapy and / or stem cell or bone marrow transplants. Knips et. al compared exercise as an addition to standard therapy with standard therapy alone: the evidence is very uncertain about the effect of exercise on anxiety and serious adverse events. Exercise may cause little or no change in mortality, quality of life, and physical function. Exercise may cause a small reduction in depression.
literature
- Review article
- JE Rubnitz, H. Inaba: Childhood acute myeloid leukaemia. In: British Journal of Hematology . Volume 159, Number 3, November 2012, pp. 259-276, ISSN 1365-2141 . doi: 10.1111 / bjh.12040 . PMID 22966788 . PMC 3468705 (free full text). (Review).
- U. Creutzig, MM van den Heuvel-Eibrink, B. Gibson, MN Dworzak, S. Adachi, E. de Bont, J. Harbott, H. Hasle, D. Johnston, A. Kinoshita, T. Lehrnbecher, G. Leverger , E. Mejstrikova, S. Meshinchi, A. Pession, SC Raimondi, L. Sung, J. Stary, CM Zwaan, GJ Kaspers, D. Reinhardt: Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. In: Blood . Volume 120, Number 16, October 2012, pp. 3187-3205, ISSN 1528-0020 . doi: 10.1182 / blood-2012-03-362608 . PMID 22879540 . (Review).
- B. Löwenberg, JR Downing, A. Burnett: Acute myeloid leukemia. In: The New England journal of medicine . Volume 341, Number 14, September 1999, pp. 1051-1062, ISSN 0028-4793 . doi: 10.1056 / NEJM199909303411407 . PMID 10502596 . (Review).
- Guidelines
- S1 guideline for acute myeloid leukemia (AML) in childhood of the Society for Pediatric Oncology and Hematology (GPOH). In: AWMF online (as of 2013)
- Others
- JM Bennett, D. Catovsky, MT Daniel, G. Flandrin, DA Galton, HR Gralnick, C. Sultan: Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. In: Annals of internal medicine . Volume 103, Number 4, October 1985, pp. 620-625, ISSN 0003-4819 . PMID 3862359 .
- JW Vardiman, NL Harris, RD Brunning: The World Health Organization (WHO) classification of the myeloid neoplasms. In: Blood. Volume 100, Number 7, October 2002, pp. 2292-2302, ISSN 0006-4971 . doi: 10.1182 / blood-2002-04-1199 . PMID 12239137 . (Review).
Web links
- Competence Network Pediatric Oncology and Hematology (KPOH) /kinderkrebsinfo.de: Acute Myeloid Leukemia (AML) doi: 10.1591 / poh.patinfo.aml.1.20060414 .
- Onkodin - data and information on oncology and hematology
- Competence Network Acute and Chronic Leukemia (KNL): Acute Myeloid Leukemia (AML)
Individual evidence
- ↑ fusion gene fusion gene
- ↑ Prescribing Information Vidaza . March 2016.
- ↑ Yasemin Küley-Bagheri, Karl-Anton Kreuzer, Ina Monsef, Michael Lübbert, Nicole Skoetz: Effects of all-trans retinoic acid (ATRA) in addition to chemotherapy for adults with acute myeloid leukaemia (AML) (non-acute promyelocytic leukaemia ( non-APL)) . In: Cochrane Database of Systematic Reviews . August 6, 2018, doi : 10.1002 / 14651858.CD011960.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ Lise Estcourt, Simon Stan Worth, Carolyn Doree, Sally Hopewell, Michael F Murphy: Prophylactic platelet transfusion for prevention of bleeding in patients with Haematological disorders after chemotherapy and stem cell transplantation . In: Cochrane Database of Systematic Reviews . May 16, 2012, doi : 10.1002 / 14651858.CD004269.pub3 ( wiley.com [accessed July 9, 2020]).
- ↑ Lise J Estcourt, Simon J Stanworth, Carolyn Doree, Sally Hopewell, Marialena Trivella: Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation . In: Cochrane Database of Systematic Reviews . November 18, 2015, doi : 10.1002 / 14651858.CD010983.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ Sheila A Fisher, Antony Cutler, Carolyn Doree, Susan J Brunskill, Simon J Stanworth: Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition . In: Cochrane Database of Systematic Reviews . January 30, 2019, doi : 10.1002 / 14651858.CD009768.pub2 ( wiley.com [accessed July 9, 2020]).
- ^ Haematologica November 2011
- ↑ Linus Knips, Nils Bergenthal, Fiona Streckmann, Ina Monsef, Thomas Elter: Aerobic physical exercise for adult patients with haematological malignancies . In: Cochrane Database of Systematic Reviews . January 31, 2019, doi : 10.1002 / 14651858.CD009075.pub3 ( wiley.com [accessed July 9, 2020]).