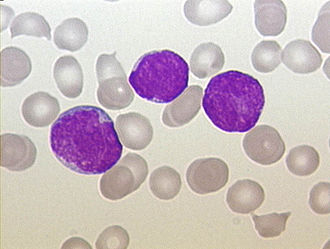
Acute lymphoblastic leukemia
| Classification according to ICD-10 | |
|---|---|
| C91.0 | Acute lymphoblastic leukemia ICD-O 9835/3 (B-line) ICD-O 9837/3 (T-line) |
| ICD-10 online (WHO version 2019) | |
The acute lymphoblastic leukemia (syn. Acute lymphoblastic leukemia , short ALL ) is an acute leukemia , the degenerated from malignant precursor cells of lymphocytes runs out. This usually leads to rapidly progressing bone marrow insufficiency (decreased bone marrow function ), i. H. a weakening healthy blood production with a lack of erythrocytes (red blood cells) and thrombocytes (blood platelets). This is accompanied by an increasing general weakness and tendency to bleed. The number of leukocytes (white blood cells) in the blood may initially be increased, normal or even reduced. Due to the relative lack of healthy, functional leukocytes, there is also an immunodeficiency with often severe and u. U. life-threatening infections. The treatment takes place by means of chemotherapy and z. T. also radiation therapy . While ALL 30–40 years ago led to death in the vast majority of patients within a few weeks, today it can be cured with intensive chemotherapy in over 50% of adults and around 80% of all children. The individual chances of recovery depend heavily on the presence of certain risk factors.
frequency
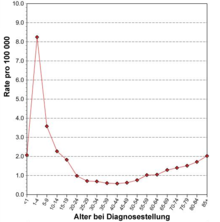
ALL is a rare disease with an incidence of around 1.5 new cases / 100,000 per year based on all age groups. Male patients are overweight (1.4 to 1). For Germany it is estimated about 500 new cases in adults and about 500 new cases in children each year. Exact figures do not exist for adults due to the lack of a central cancer registry ; the figures for children under the age of 15 are based on data from the German Childhood Cancer Registry , in which an estimated 90% of all cases are recorded. In Switzerland and Austria , around 40–50 new cases are assumed in adults and children each year. In the United States , the number of new cases in 2007 is estimated to be approximately 5200. The lifetime risk of developing acute lymphoblastic leukemia is therefore around 1 in 838, i.e. H. about one person under 838 will develop ALL in their lifetime. The distribution across the age groups shows a peak in childhood (6.5 / 100,000 in children under 4 years of age) and a second, lower peak in older age (1.5 / 100,000 in those over 80). In adults, ALL accounts for less than 15% of all acute leukemias (i.e., more than 85% of all diseases are acute myeloid leukemias ). This is the other way around in children. ALL is the most common malignant disease in childhood.
Causes and origins
The cause of the disease are genetic changes in a lymphatic cell that lead to the malignant (malignant) transformation of this cell. These genetic changes (apart from rare special cases) are acquired in the course of life and are neither inherited nor inheritable, since the germ line cells (egg cells, sperm) are not affected. The exact causal chain that leads to the occurrence of the disease is not yet known. There are risk factors for the development of leukemia ( ionizing radiation , chemical mutagens, etc.), but in the vast majority of patients, despite careful research, no specific cause can be found. So far there is also no evidence of a possible infectious cause, e.g. B. by viruses . The only exception is adult T-cell leukemia in Japan , which is caused by the retrovirus HTLV-1 , but which is virtually non-existent in Europe. The malignant transformed cell and its daughter cells, which are produced by cell division , multiply in an uncontrolled and unchecked manner and suppress normal blood formation (hematopoiesis) in the bone marrow . The ALL is a clonal disease, i. H. all ALL cells are nearly identical genetic copies of one another. The malignant cells mainly accumulate in the lymphatic organs (especially lymph nodes , spleen , thymus ), but also in other organs such as B. the central nervous system (CNS). There is an increasing disturbance of blood formation (bone marrow insufficiency) with anemia ( anemia ) and thrombocytopenia (lack of platelets ) and an associated bleeding tendency as well as an immunodeficiency . If left untreated, the disease is quickly fatal.
Symptoms
The symptoms of ALL are similar to those of acute myeloid leukemia .
It occurs:
- Blood formation disorders:
- Anemia with general weakness, poor performance, fatigue
- Lack of thrombocytes (blood platelets), u. U. associated with a tendency to bleed, e.g. B. Spontaneous bleeding of any kind
- Severe immune deficiency due to a relative lack of functional white blood cells (leukocytes) with severe infections
- often hepatosplenomegaly (enlarged liver and spleen )
- frequent swelling of the lymph nodes (although not as pronounced as in chronic lymphocytic leukemia )
- sometimes bone pain, which can also be the first symptom of the disease (especially in children)
- in approx. 10% of the cases meningeosis leucaemica (attack of the central nervous system by leukemia cells) u. U. with neurological deficits
- Thymus swelling (so-called mediastinal tumor ) often in T-ALL with u. U. upper influence stagnation
At the time of diagnosis, children with ALL show the following relative frequencies of clinical signs, symptoms and typical laboratory findings. The latter reflect the extent of the disruption of blood formation caused by ALL, since normal blood formation in the bone marrow is suppressed by ALL (so-called "displacement myelopathy").
| Clinical signs and symptoms in children with acute lymphoblastic leukemia | ||
|---|---|---|
| Symptoms | Proportion of patients | |
| Fever (temperature> 38.5 ° C) | 61% | |
| Signs of bleeding (e.g., petechiae or purpura ) | 48% | |
| Bone pain | 23% | |
| Swelling of the lymph nodes ( lymphadenopathy ) | 50% | |
| Sole enlargement of the spleen ( splenomegaly ) | 63% | |
| Enlargement of the spleen and liver ( hepatosplenomegaly ) | 68% | |
| Laboratory test results in children with acute lymphoblastic leukemia | ||
|---|---|---|
| Laboratory test results | Range of values | Proportion of patients |
| Leukocytes; WBC (white blood cells) , "Leukos" | Normal: 4,000–12,000 / µL * | |
| <10,000 / µL | 53% | |
| 10,000 to 49,000 / µL | 30% | |
| > 50,000 / µL | 17% | |
| Hemoglobin; HGB, Hb | Normal: 11.0-16.0 g / dL * | |
| <7.0 g / dL | 43% | |
| 7.0 to 11.0 g / dL | 45% | |
| > 11.0 g / dL | 12% | |
| Platelets; PLT (platelets) , "Thrombos" | Normal: 150,000–450,000 / µL * | |
| <20,000 / µL | 28% | |
| 20,000 to 99,000 / µL | 47% | |
| > 100,000 / µL | 25% | |
| * The standard values for childhood represent an approximate range of values. If standard ranges are used exactly, these must be differentiated according to age groups (newborns, infants, toddlers, school children, adolescents). This is especially true for the concentration of hemoglobin and the number of leukocytes. This differentiation is dispensed with here to illustrate the relationships. | ||
| Laboratory test results in adults with acute lymphoblastic leukemia | ||
|---|---|---|
| Laboratory test results | Range of values | Proportion of patients |
| Leukocytes; WBC (white blood cells) , "Leukos" | Normal: 4,000–11,000 / µL * | |
| <5,000 / µL | 27% | |
| 5,000 to 10,000 / µL | 14% | |
| > 10,000 / µL | 59% | |
| Hemoglobin; HGB, Hb | Normal: 11.0–16.0 g / dL (women), 13.0–18.0 (men) * | |
| <8.0 g / dL | 28% | |
| 8.0 to 12.0 g / dL | 51% | |
| > 12.0 g / dL | 21% | |
| Platelets; PLT (platelets) , "Thrombos" | Normal: 150,000–450,000 / µL * | |
| <25,000 / µL | 30% | |
| 25,000 to 150,000 / µL | 55% | |
| > 150,000 / µL | 15% | |
| * The normal values for adults vary slightly depending on the laboratory. At the time of diagnosis, 92% of patients had blasts in the peripheral blood. | ||
Diagnostics and classification

An examination of the bone marrow is essential for the diagnosis , since it can happen that at the time of diagnosis there is no detectable flushing of leukemia cells from the bone marrow into the blood. One then speaks of an aleukemic form (see the two tables on laboratory test results). If leukemia cells are detectable in the blood, but the total number of leukocytes is not increased, one speaks of a subleukemic course. When the total number of leukocytes is increased by leukemia cells, this is called a leukemic course. A normal or even reduced leukocyte count in the blood does not rule out leukemia; bone marrow diagnostics are decisive.
The diagnosis of ALL can be made by:
- Detection of a proportion of lymphatic blasts of at least 20 to 25% in the bone marrow
- Allocation of the blasts to the lymphatic series by immunophenotyping (see below)
- Evidence of characteristic genetic changes (see below)
Cytomorphology
The diagnosis of acute leukemia can be made by microscopic examination of the bone marrow or the blood smear in the case of leukemic disease. In contrast to AML, the assessment of the cell morphology only plays a subordinate role for the further subclassification of ALL. In the so-called FAB classification ("French-American-British") a distinction is made between three different morphologies (L1, L2, L3). This is only important for the rare L3 subtype that is associated with “mature-cell B-ALL”. Mature-cell B-ALL is a special form of ALL and can be viewed and treated as the leukemic manifestation of Burkitt's lymphoma (i.e., Burkitt's lymphoma with> 20% or> 25% bone marrow involvement). The distinction between L1 and L2 morphology, on the other hand, is difficult and even very experienced hematologists or hematopathologists come to different assessments here. The distinction between L1 and L2 has no clinical significance.
| Frequency of cytomorphological findings according to FAB classification in children with ALL | ||
|---|---|---|
| FAB cytomorphological class | Characteristics | Proportion of patients |
| FAB L1 | Small cells predominantly of uniform size, uniform chromatin, cell nucleus not visible or small, nucleoli regularly found, very little cytoplasm, slight to moderate basophilia, varying degrees of vacuoles in the cytoplasm | 84% |
| FAB L2 | Large cells of different sizes, irregular chromatin, cell nucleus with irregular cracks and notches, one or more often large nucleoli, variable size of the cytoplasm, moderate to often severe basophilia, varying degrees of vacuoles in the cytoplasm | 15% |
| FAB L3 | Large cells of uniform size, uniformly finely speckled chromatin, oval to round nucleus, protruding nucleoli (even several), a lot of cytoplasm, very strong basophilia, often prominent vacuoles in the cytoplasm | 1 % |
Immunophenotyping
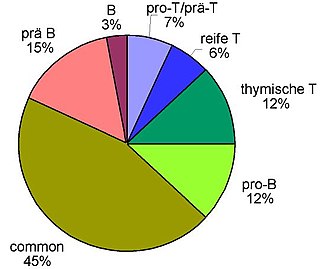
The decisive factor is the immunophenotyping of the leukemia cells obtained from the blood or by means of bone marrow puncture , which today is usually carried out using flow cytometry (FACS = fluorescence activated cell sorting ). This is used to investigate whether and to what extent certain proteins are located on the surface or in the cytoplasm of cells. The expression pattern of different lymphatic, myeloid and precursor cell antigens enables the assignment to B or T cell series and the determination of the differentiation stage. The ALL can accordingly be classified as B-line ALL (with B-lymphocytic differentiation, further subdivided into "B-precursor ALL" and "mature (cellular) e B-ALL") or T-line ALL (with T- lymphocytic differentiation, further subdivided into "T-precursor ALL" and "mature T-ALL"). The table below shows the classification of acute lymphoblastic leukemia based on the surface antigen pattern according to the so-called EGIL classification (EGIL = European Group for the Immunological Characterization of Leukemias ). The ALL cells are classified according to their "degree of maturity", i. H. a pro-B-ALL is a completely immature form , followed by the common ALL , followed by the pre-B-ALL . The mature B-ALL already shows many characteristics of mature B-lymphocytes in its antigen pattern. It is analogous for the T series: pro-T → pre-T → thymic (cortical) T → mature T. About 75% of ALLs in adulthood can be assigned to the B-lymphocyte series and about 25% to the T-lymphocyte series . With ALL in childhood and adolescence, the ratio is around 85%: 15%.
| Immunological classification of acute lymphoblastic leukemia | ||||||||
|---|---|---|---|---|---|---|---|---|
| B-line ALL | T-line ALL | |||||||
| pro-B | common | pre-B | maturity | pro-T | pre-T | cortical (thymic) | maturity | |
| B cell antigens | ||||||||
| CD19 | + | + | + | + | - | - | - | - |
| cyCD22 | + | + | + | + | - | - | - | - |
| CD79alpha | + | + | + | + | - | - | - | - |
| cyIgM | - | - | + | - | - | - | - | - |
| mIg | - | - | - | + | - | - | - | - |
| T cell antigens | ||||||||
| cyCD3 | - | - | - | - | + | + | +/- | - |
| CD7 | - | - | - | - | + | + | + | + |
| CD2 | - | - | - | - | - | + | + | + |
| CD1a | - | - | - | - | - | - | + | - |
| mCD3 | - | - | - | - | - | - | +/- | + |
| Precursor cell antigens | ||||||||
| TdT | + | + | + | - | + | + | + | +/- |
| HLA-DR | + | + | + | + | +/- | - | - | - |
| CD10 | - | + | +/- | +/- | +/- | +/- | +/- | - |
Cytogenetics and Molecular Genetics
The cytogenetics and molecular genetics of acute leukemia are the subject of intensive research . In the last two decades a great deal of work has been published that deals with various aspects of the genetics of these diseases. To a certain extent, genetics form the basis for a deeper understanding of the disease.
Basically, it can be said that the ALL of childhood or infancy, as far as the genetic basis is concerned, differs in some cases considerably from the ALL of adulthood. Therefore, the three age groups should be considered separately here:
ALL in infancy (less than one year)
Characteristic of ALL in infants (English infant leukemia ) are changes in the MLL gene on the long arm of chromosome 11 , band 23 (“11q23 aberrations”). These changes are associated with an unfavorable prognosis. The MLL gene is usually fused with other genes through chromosome translocations. To date, over 50 merger partners are known. By far the most common aberration is the fusion with the AF4 gene on chromosome 4 (more rarely also ENL gene on chromosome 19 , AF9 gene on chromosome 9 ). This creates a so-called fusion protein (“ chimeric ” protein), so that the actual functions of the genes involved are either lost or changed to such an extent that they cause or promote disease.
ALL in childhood (1 to 15 years)
A multitude of genetic changes have been found in childhood ALL. In simplified terms, the following groups are currently differentiated genetically:
- Patients with a hyperdiploid chromosome set (i.e. chromosome multiplication ) - prognostically favorable
- Patients with a hypodiploid set of chromosomes (i.e. reduction in chromosomes) - prognostically unfavorable
- Patients with the chromosome translocation t (12; 21), which leads to the fusion gene TEL-AML1 - prognostically favorable
- Patients with the chromosome translocation t (9; 22), which leads to the fusion gene BCR-ABL - prognostically unfavorable so far, whereby the additional administration of imatinib (Glivec) has opened up a new treatment approach which, according to the first study results, significantly improves the prognosis
- Patients with the chromosome translocation t (1; 19), which leads to the fusion gene E2A-PBX1 - prognostically favorable
- Patients with MLL aberrations (see above) - prognostically unfavorable
- Patients with T-line ALL - prognostically unfavorable
The most common change is the t (12; 21), which is found in around 15–20% of all cases.
ALL in adulthood (from 16 years)
In principle, the same genetic changes are found in adults as in children, only with considerably different frequencies. The most frequent change is the t (9; 22), which can be found in approx. 25% of the cases. The t (12; 21), on the other hand, is rare (approx. 1%). The prognostic value of the above Changes in adults are e.g. Sometimes not yet clearly clarified (t (12; 21), t (1; 19)). In contrast to the situation in children, T-ALL is not generally considered to be prognostically unfavorable.
treatment
Prognostic factors
ALL is not a uniform clinical picture, but can take a very different course in different patients. Some patients show e.g. B. a good response to therapy, while others respond only delayed to therapy or relapse quickly . Various risk factors have been identified over the past few decades. In this context, risk factors are factors that result in the affected patient having an increased risk of responding poorly to treatment or of suffering a relapse.
ALL is not treated uniformly worldwide. There are various large so-called study groups in different countries who treat their patients according to certain therapy schemes. In the meantime, however, there are efforts to align the therapies with one another or to initiate cross-border therapy studies (e.g. within the framework of the European LeukemiaNet ).
Examples of such study groups are:
Study groups for adult patients
- GMALL (German Multicenter ALL Study Group) - in Germany and some other countries
- GIMEMA (Gruppo Italiano Malattie Ematologiche dell 'Adulto) - in Italy
- MRC (Medical Research Council) - in the UK
- PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatía Maligna) - in Spain
- GRAALL (Group for Research on Adult Acute Lymphoblastic Leukemia) - in France , Belgium and Switzerland
- NILG (Northern Italy Leukemia Group) - in Northern Italy
- PALG (Polish Adult Leukemia Group) - in Poland
- SWOG (Southwest Oncology Group) - in the USA
Study groups for children and adolescents
- AIEOP-BFM-ALL , successor to ALL-BFM , Berlin-Frankfurt-Münster cooperative multicenter ALL study - for primary disease - Germany, Austria, Switzerland, e.g. T. also other European countries
- COALL Cooperative ALL Study - for Primary Disease - Germany
- ALL-REZ BFM Berlin-Frankfurt-Münster cooperative multicenter study ALL recurrence - for relapse - Germany, Austria, Switzerland
- ALL-SZT BFM Berlin-Frankfurt-Münster cooperative multicenter study ALL stem cell transplantation - Germany, Austria, Switzerland
- I-BFM (International BFM-Group) - treatment protocol based on ALL-BFM - Europe
- COG (Children's Oncology Group) - various protocols - USA
- UKALL (United Kingdom ALL Study Group) - UK Children's Cancer Study Group Treatment Protocol - United Kingdom
- SFOP (Societé française d'oncologie pédiatrique) - France
- NOPHO (Nordic Society of Pediatric Hematology and Oncology) - Scandinavia
Established risk factors
Almost all study groups were able to identify the following risk factors - although the respective therapy is different:
- 1. Genetic detection of the BCR-ABL fusion gene, often (but not always) cytogenetically visible as the " Philadelphia chromosome "
- 2. high peripheral white blood cell count at diagnosis as an expression of a high " tumor burden " (tumor burden)
- 3. Delayed response to therapy (especially after the induction phase of therapy)
- 4. Genetic detection of an MLL fusion gene
- 5. the patient's age: young patients usually have significantly better chances of recovery than older ones (exception: small children under 1 year of age)
- 6. Involvement of the central nervous system (brain and spinal cord) by ALL
- 7. Certain immune phenotypes (e.g. T-line ALL in children and adolescents (1–18 years))
Other risk factors are controversial and not widely accepted. It must also be emphasized that the above factors are statistical risk factors, i. H. In individual cases, the clinical course can also look different than expected. The identification of risk factors is important because the affected patients are high-risk patients who are at great risk of relapse . This is why the previous therapy concepts primarily provide for more intensive treatment for these patients. In general, adults in first remission , the allogeneic bone marrow - or stem cell transplantation sought. In children with ALL, however, a stem cell transplant is only carried out if special and rare risk factors indicate such a condition, or if ALL recurs after remission (relapses).
Graft-versus-host reactions
In cases where a stem cell transplant is required, a graft-versus-host reaction can occur. In order to treat or avoid this, mesenchymal stromal cells can be useful. In 2019, Fisher et al. created a Cochrane review of randomized controlled trials to measure the safety and efficacy of mesenchymal stromal cells (MSC) in patients with a graft-versus-host reaction (GvHD). The patients suffered from this condition because they received a blood stem cell transplant as a treatment for a haematological disease. Fisher et al. included studies that used MSCs for either therapeutic or prophylactic purposes. The exact inclusion and exclusion criteria and information regarding the dose can be found in the original Cochrane review article. In the therapeutic studies, the patients had to suffer from GvHD. Fisher et al. conducted an analysis: MSCs compared with a control treatment (e.g. placebo) for immune-mediated inflammation after transplantation and in autoimmunity: The evidence is very uncertain about the effect of MSCs on the complete remission of acute and chronic GvHD and all-cause mortality, if they are used for therapeutic reasons. MSCs achieve little or no change in all-cause mortality, recurrence of malignancy, and the incidence of acute GvHD when used prophylactically. MSCs may reduce the incidence of chronic GVHD when used for prophylactic purposes.
Minimal residual disease
As minimal residual disease (MRD short, minimal residual disease ) refers to the percentage of leukemic cells during or after therapy. One speaks z. B. from an MRD of 0.001% or 10 −5 , if one leukemia cell can still be found out of 100,000 healthy bone marrow cells . It is now known that the MRD is the most important prognostic factor in the treatment of leukemia. As part of modern leukemia therapy concepts, the MRD is therefore also regularly determined in order to determine the degree of therapy response. This then serves at the same time for stratification , i.e. H. the treated patients are given different additional forms of treatment depending on the course of the MRD. The goal is to give as much chemotherapy as needed.
The MRD is usually determined using the polymerase chain reaction (PCR). This requires highly specialized laboratories that are experienced in this particular diagnostics. If the therapy does not succeed in bringing the MRD below a certain level (at least 10 −4 ), a relapse of the disease is practically predetermined.
chemotherapy
Various chemotherapy procedures can be used to treat ALL.
Before starting the treatment, a detailed diagnosis must be carried out in order to characterize the ALL genetically and immunocytologically (see above). This also includes an examination of the cerebrospinal fluid by lumbar puncture and possibly imaging of the central nervous system (for example by magnetic resonance imaging ). Treatment is started immediately after the diagnosis. This is done using chemotherapy, i. H. by administering cytostatics . Combinations of different cytostatic agents are given, as this increases the antileukemic effect many times over. Treatment lasts at least 12 to 24 months in total.
In simple terms, one can say that the treatment proceeds according to the following scheme:
Induction therapy (possibly as "double induction") → consolidation therapy → re-induction therapy → maintenance therapy
Induction phase (1–3 months)
At the beginning there is the "induction therapy", which is very intensive and rich in side effects. The most important cytostatics are in this phase
- Corticosteroids , e.g. B. dexamethasone and prednisone (strictly speaking do not belong to the group of cytostatic drugs, but corticosteroids inhibit the reproduction of lymphatic immune cells and thus usually also the reproduction of ALL cells)
- Anthracyclines , e.g. B. daunorubicin or doxorubicin
- Antimetabolites , e.g. B. Cytarabine
- Vinca alkaloids , e.g. B. Vincristine
- Alkylating agents , e.g. B. Cyclophosphamide
- L-asparaginase
Defined cytostatic drugs are administered to the patient in predetermined doses and combinations on precisely defined days of the treatment process. In the AIEOP BFM ALL 2009 study in children, the induction phase lasts 9 weeks with an uncomplicated course. Its aim is to suppress ALL in such a way that one month after the start of the induction phase it is hardly detectable in the bone marrow puncture and at the end of the induction phase it is no longer detectable. The reason the treatment is so intensive is that one does not want to give the blasts time to develop resistance to cytostatics and therefore wants to reduce the tumor burden as quickly as possible .
Occasionally, after the first induction therapy, a second induction therapy is added ("double induction"), which contains somewhat different drug combinations and also lasts about a month.
Consolidation (several months)
Although ALL can ideally no longer be detectable after the induction phase has ended, treatment is continued. Stopping treatment at this point in time would otherwise very likely lead to a relapse: ALL is hardly or no longer detectable with the diagnostic methods available, but has not yet been completely eliminated. The “consolidation phase” follows the induction phase. In this phase, too, cytostatics are administered at fixed doses and at fixed intervals. The most important cytostatics in the consolidation phase are:
- Methotrexate
- Cyclophosphamide
- Cytarabine
- Etoposide or earlier also teniposide
- L- asparaginase
- Tioguanine and / or 6-mercaptopurine
In particular, the cytostatics methotrexate and possibly also cytarabine are used in pulses in medium to high doses. Tioguanine and / or 6-mercaptopurine and / or L- asparaginase are added as long-term therapy, with asparaginase being given a dose every two weeks due to its slow degradation, in order to keep it permanently in the body.
Reinduction
The “re-induction phase” follows the consolidation phase. In this phase, the treatment is intensified again as in the induction phase. The cytostatic agents used correspond to those of the induction phase.
Induction phase, consolidation phase and re-induction phase are also referred to as the intensive phase of treatment. The total duration of the intensive phase depends on the risk factors and the course of treatment of the affected patient. The duration varies between 6 and 12 months. In-patient hospitalization is usually necessary in the intensive phase when administering cytostatic drugs and when complications such as infections in bone marrow aplasia (“cell deep”) occur. The patient stays at home between the individual cytostatics cycles without any problems; only outpatient checks of the blood count are necessary and useful.
Maintenance therapy
In the “maintenance phase”, which again immediately follows the intensive phase, the patients receive chemotherapy with methotrexate and 6-mercaptopurine . In contrast to the previous treatment phases, chemotherapy in this phase is usually carried out as oral chemotherapy (in the form of tablets): 6-mercaptopurine is then taken once a day, methotrexate once a week. In contrast to the intensive phase, hospitalization is not required, except in the event of complications. The duration of the maintenance phase varies depending on the therapy protocol used and is 6–18 months. In children and adolescents, maintenance therapy with 6-mercaptopurine and methotrexate is typically carried out up to two years after the diagnosis or the start of treatment.
CNS prophylaxis, CNS irradiation
A problem with most cytotoxic drugs is that they do not cross the blood-brain barrier at all or only poorly . If leukemia cells penetrate the central nervous system (CNS), they are protected there from cytostatics and from there can lead to a relapse of the disease. In order to prevent such CNS relapses, a more or less intensive so-called CNS prophylaxis is carried out in all patients. This can be done in two forms. On the one hand as multiple repeated direct administration of cytostatics (usually methotrexate) into the CSF space ("nerve water") by means of lumbar puncture (= intrathecal administration), on the other hand as irradiation of the central nervous system. CNS irradiation was standard, especially in the first decades of ALL treatment. In children and adolescents, CNS irradiation has increasingly been replaced by intrathecal administration of methotrexate for most patients due to the observed late effects. However, if the central nervous system is definitely affected at the beginning of treatment or if certain risk factors are met, such as an initial leukocyte count of more than 100,000 per microliter in the peripheral blood, irradiation of the central nervous system is still carried out even in children.
Mediastinal irradiation
If there is strong thymus swelling caused by leukemia cells as a so-called mediastinal tumor , this is locally irradiated in adults (in children and adolescents only in exceptional cases, since the thymus in children, unlike adults, still fulfills essential functions in the immune system).
Control of the therapy success
Another bone marrow puncture is performed at regular intervals to check the success of the therapy. Regular checks of the nerve fluid (liquor) for the presence of ALL cells are also essential. As a rule, induction therapy, which usually lasts about 2–3 months (including the aplasia times, see the following section), reduces the “leukemia tumor burden” by at least a factor of 1,000 to 10,000 or more.
Blood count check
The treatment cannot be as intensive and highly dosed as desired, since the cytostatic drugs have considerable side effects that are dose-limiting. The most important side effect is bone marrow depression , i.e. damage to the remaining healthy blood formation , which is already severely weakened in leukemia sufferers . As a result of the chemotherapeutic treatment, aplasia occurs for a long time , i. H. an almost complete failure of normal blood formation. In this phase, which can last up to a month or longer, missing blood components have to be replaced by transfusions and the patient is extremely at risk of infection due to the lack of immune defense . Quite a few patients die during treatment from severe infections acquired in aplasia.
In 2012 and 2015, Estcourt et al. Cochrane reviews of randomized controlled trials designed to find out which use of platelet transfusions is most effective for preventing bleeding in patients with haematological disorders when receiving chemotherapy (or a stem cell transplant).
Treatment outcomes
Before effective cytotoxic drugs were available, the diagnosis of “acute lymphoblastic leukemia” was practically the death sentence for the affected patient. Depending on the stage of the disease, those affected died within days to weeks of the diagnosis. The main causes of death were severe infections due to severe immunodeficiency , spontaneous bleeding due to thrombocytopenia or other complications (e.g. if the central nervous system was affected ). Particularly tragic and serious was the fact that small children were often affected by the disease (ALL is the most common malignant disease in childhood). At the end of the 1970s, when some effective cytotoxic drugs were already available, the mean 5-year survival in adult ALL patients in Germany was less than 15%. The results were better in children.
In the period that followed, intensive efforts were made to improve the therapeutic results for ALL patients. This was done through extensive sequential clinical trials in which the patients were treated. The results and experiences from one study were then used to plan a new, subsequent improved therapy study. Overall, overall survival in ALL patients is currently around 80% in children and around 40–45% in adults. These numbers vary as they depend on the particular subtype and therapy. Further improvements can be expected in the future. It must be emphasized, however, that these numbers represent mean values. In individual cases, the prognosis can differ significantly. So z. B. a 20-year-old patient without risk factors (see above) expect significantly better chances of recovery than z. B. a 65 year old patient with risk factors.
Supportive therapy options
In addition to aggressive treatment of the underlying disease, it can also make sense to improve the patient's well-being. Physical activity is often mentioned in this context. To evaluate the feasibility and effectiveness, Knips et al. prepared a Cochrane review article in 2019. There, physical activity was studied in addition to standard therapy in patients suffering from a malignant haematological disease. The exact inclusion and exclusion criteria and further details can be found in the original Cochrane review. The study participants had disease stages I to IV. Since physical activity was only an additional treatment, the patients also received chemotherapy and / or stem cell or bone marrow transplants. Knips et. al compared exercise as an addition to standard therapy with standard therapy alone: the evidence is very uncertain about the effect of exercise on anxiety and serious adverse events. Exercise may cause little or no change in mortality, quality of life, and physical function. Exercise may cause a small reduction in depression.
Future developments
Targeted drugs offer great hope for the future. These are drugs that have a much more specific effect on leukemia cells than conventional cytostatics and thus have a less serious side effect profile. Examples of such substances are the active ingredients Imatinib ( Glivec ® ), Nilotinib ( Tasigna ® ) and Dasatinib ( Sprycel ® ), which are used in studies in addition to standard chemotherapy in Philadelphia chromosome- positive ALL. Also, monoclonal antibodies such as rituximab or alemtuzumab or the bispecific antibody Blinatumomab will influence the future therapeutic regimens of ALL.
In fact, L- asparaginase , which has been part of the standard repertoire of chemotherapeutic agents used against ALL for decades, is also a targeted drug : It splits an amino acid in the blood , namely asparagine , which normal body cells can produce themselves from other amino acids, but ALL cells as a rule Not. Thus, L-asparaginase in the blood effectively starves the leukemia cells and at the same time has few side effects on the other cells. However, the originally used L-asparaginase often caused allergies in the patient, with the result that it could not be passed on or was made ineffective by the allergic defense of the patient's body anyway. Modifications ( PEG- L-asparaginase, Erwinase ) should help to increase the proportion of patients who can be given asparaginase over a long period of time.
history
The term “ leukemia ” was coined by Rudolf Virchow , who in 1845 described the clinical picture of leukemia (probably chronic myeloid leukemia ) almost simultaneously with and independently of the Scottish pathologist John Hughes Bennett and the French Alfred Donné . In the following years Virchow developed his concept of "cellular pathology", which became fundamental for pathology. In 1868 Ernst Neumann first described the bone marrow as a place where blood cells were formed and later also developed the stem cell theory of hematopoiesis . After the advent of synthetic chemical dyes, Paul Ehrlich developed techniques for staining blood smears in 1877 , which made it possible to distinguish more precisely from leukocytes. Wilhelm Ebstein coined the term “acute leukemia” in 1889 to differentiate it from chronic leukemia. In 1879, Mosler first described the techniques of bone marrow examination to diagnose leukemia. In 1900 Otto Naegeli introduced the classification of leukemia into “myeloid” and “lymphatic”.
The first real therapeutic success in the sense of a (temporary) remission of the disease could only be achieved after the development of the first cytostatics. In 1948 Sidney Farber in Boston was able to induce temporary remissions in children with acute lymphoblastic leukemia by administering aminopterin , a folic acid antagonist. In the 1950s, further cytostatics effective in ALL were developed or discovered: 6-mercaptopurine by George Hitchings and Gertrude Elion , the vinca alkaloids by Robert Laing Noble and the glucocorticoids. However, the first long-term survivors did not exist until combination therapies with various cytostatics had been tried out systematically. The St. Jude Children's Research Hospital, founded in Memphis / Tennessee in 1962, was groundbreaking . Under its first director Donald Pinkel , the Total Therapy studies, which included a combination therapy of various cytostatics with CNS prophylaxis (intrathecal drug administration or CNS irradiation), were carried out here. In the 1970s, the beginnings of what is today called BFM study group were ( B erlin- F rankfurt- M ünster), which today is the world's largest treatment study group for children. A pioneer in this field in Europe was Hansjörg Riehm (initially in Berlin, later at the Hannover Medical School). At the end of the 1970s, there were the first large-scale multicenter therapy studies on ALL in adults.
literature
- CH Pui, MV Relling, JR Downing: Acute lymphoblastic leukemia. In: N Engl J Med . 2004, 350, pp. 1535-1548. PMID 15071128
- CH Pui, WE Evans: Treatment of acute lymphoblastic leukemia. In: N Engl J Med. 2006, 354, pp. 166-178. PMID 16407512
- N. Gökbuget (Ed.): Acute lymphatic leukemia. 1st edition. Verlag UNI-MED Science, 2007, ISBN 978-3-89599-218-6 .
Web links
- kinderkrebsinfo.de
- German Multicenter Study Group on Adult Acute Lymphoblastic Leukemia (GMALL) ( Memento from February 4, 2015 in the Internet Archive )
- Competence Network Acute and Chronic Leukemia: Acute Lymphatic Leukemia (ALL)
- European LeukemiaNet with links to study groups and treatment protocols
- Southwest Oncology Group (SWOG) in the USA
Individual evidence
- ↑ a b Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI)
- ↑ German Childhood Cancer Register
- ↑ The Association of Swiss Cancer Registries ( Memento of December 13, 2002 in the Internet Archive ) only contains data from selected cantons and, just like Statistics Austria in Austria, has recorded all leukemia diseases together, of which the ALL is of course only a fraction.
- ↑ a b c J. F. Margolin, CP Steuber, DG Poplack: Acute Lymphoblastic Leukemia. In: PA Pizzo, DG Poplack (Ed.): Principles and Practice of Pediatric Oncology. 4th edition. Lippincott Williams & Wilkins. Baltimore 2003, ISBN 0-7817-2658-1 .
- ↑ D. Hoelzer, N. Gökbuget: Acute lymphatic leukemia. In: S. Seeber, J. Schütte (Ed.): Therapy Concepts Oncology . Springer-Verlag, Heidelberg 2007, ISBN 978-3-540-28588-5 , p. 271.
- ↑ a b M. C. Bene u. a .: Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). In: Leukemia. 1995; 9 (10), pp. 1783-1786. PMID 7564526
- ↑ MW Jansen u. a .: Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. In: Leukemia. 2007; 21, pp. 633-641. PMID 17268512
- ↑ E. Forestier et al. a .: Cytogenetic findings in a population-based series of 787 childhood acute lymphoblastic leukemias from the Nordic countries. The NOPHO Leukemia Cytogenetic Study Group. In: Eur J Haematol. 2000; 64 (3), pp. 194-200. PMID 10997816
- ↑ ML Loh u. a .: Prospective analysis of TEL / AML1-positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95–01. In: Blood . 2006; 107 (11), pp. 4508-4513. PMID 16493009
- ↑ a b K. R. Schultz u. a .: Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study ( Memento of July 29, 2012 in the Internet Archive ). In: J Clin Oncol . 2009 Nov 1; 27 (31), pp. 5175-5181. Epub 2009 Oct 5. PMID 19805687
- ↑ JM Ribera et al. a .: Prognostic value of karyotypic analysis in children and adults with high-risk acute lymphoblastic leukemia included in the PETHEMA ALL-93 trial. In: Haematologica . 2002; 87 (2), pp. 154-166. PMID 11836166
- ↑ A. Möricke et al. a .: Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. In: Klin Padiatr. 2005; 217, pp. 310-320. PMID 16307416
- ↑ DM Loo u. a .: Prognostic significance of blasts in the cerebrospinal fluid without pleiocytosis or a traumatic lumbar puncture in children with acute lymphoblastic leukemia: experience of the Dutch Childhood Oncology Group. In: J Clin Oncol. 2006; 24 (15), pp. 2332-2336. PMID 16710032
- ↑ M. Clarke et al. a .: CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. In: J Clin Oncol. 2003; 21 (9), pp. 1798-1809. PMID 12721257 .
- ↑ JM Goldberg u. a .: Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. In: J Clin Oncol. 2003; 21 (19), pp. 3616-3622. PMID 14512392 .
- ↑ Sheila A Fisher, Antony Cutler, Carolyn Doree, Susan J Brunskill, Simon J Stanworth: Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition . In: Cochrane Database of Systematic Reviews . January 30, 2019, doi : 10.1002 / 14651858.CD009768.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ GM Marshall et al. a .: Importance of minimal residual disease testing during the second year of therapy for children with acute lymphoblastic leukemia. J Clin Oncol. 2003; 21 (4), pp. 704-709. PMID 12586809
- ↑ C. Nyvold et al. a .: Precise quantification of minimal residual disease at day 29 allows identification of children with acute lymphoblastic leukemia and an excellent outcome. In: Blood. 2002; 99 (4), pp. 1253-1258. PMID 11830473
- ↑ M. Brüggemann u. a .: Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. In: Blood. 2006; 107 (3), pp. 1116-1123. PMID 16195338
- ↑ T. Raff et al. a .: Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. In: Blood. 2007; 109 (3), pp. 910-915. PMID 17023577
- ↑ DO Harms u. a .: Thioguanine offers no advantage over mercaptopurine in maintenance treatment of childhood ALL: results of the randomized trial COALL-92. In: Blood. 2003; 102 (8), pp. 2736-2740. PMID 12843002
- ↑ T. Langer et al. a .: CNS late effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. In: Med Pediatr Oncol. 2002; 38 (5), pp. 320-328. PMID 11979456
- ↑ JH Laver u. a .: Effects of cranial radiation in children with high risk T cell acute lymphoblastic leukemia: a Pediatric Oncology Group report. PMID 10720128
- ↑ V. Conter u. a .: Role of cranial radiotherapy for childhood T-cell acute lymphoblastic leukemia with high WBC count and good response to prednisone. Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Münster groups. In: Leukemia. 2000; 14 (3), pp. 369-373. In: J Clin Oncol. 1997; 15 (8), pp. 2786-2791. PMID 9256120
- ↑ Lise Estcourt, Simon Stan Worth, Carolyn Doree, Sally Hopewell, Michael F Murphy: Prophylactic platelet transfusion for prevention of bleeding in patients with Haematological disorders after chemotherapy and stem cell transplantation . In: Cochrane Database of Systematic Reviews . May 16, 2012, doi : 10.1002 / 14651858.CD004269.pub3 ( wiley.com [accessed July 9, 2020]).
- ↑ Lise J Estcourt, Simon J Stanworth, Carolyn Doree, Sally Hopewell, Marialena Trivella: Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation . In: Cochrane Database of Systematic Reviews . November 18, 2015, doi : 10.1002 / 14651858.CD010983.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ Linus Knips, Nils Bergenthal, Fiona Streckmann, Ina Monsef, Thomas Elter: Aerobic physical exercise for adult patients with haematological malignancies . In: Cochrane Database of Systematic Reviews . January 31, 2019, doi : 10.1002 / 14651858.CD009075.pub3 ( wiley.com [accessed July 9, 2020]).
- ↑ R. Virchow: The leukemia. In: R. Virchow (Ed.): Collected treatises on scientific medicine. Meidinger, Frankfurt 1856, p. 190.
- ↑ R. Virchow: Weisses Blut. In: Froriep ’s notes from the field of natural science and medicine. 1845; 36, pp. 151-156.
- ^ JH Bennett: Case of hypertrophy of the spleen and liver, in which death took place from suppuration of the blood. In: Edinburgh Medical and Surgical Journal. 1845,64, pp. 413-423.
- ↑ A. Donné: Cours de Microscopie complémentaire des études médicales. Anatomie microscopique et physiologie des fludes de l'Economie. Ballière, Paris 1844.
- ^ L. Degos: John Hughes Bennett, Rudolph Virchow ... and Alfred Donné: the first description of leukemia. In: The Hematology Journal. (2001) 2, p. 1. Full text
- ↑ E. Neumann: About the importance of the bone marrow for blood formation . Preliminary communication. In: Centralblatt for the Medical Sciences. No. 44 (1868)
- ^ W. Ebstein: About the acute leukemia and pseudoleukemia. In: Deutsch Arch Klin Med. 1889, 44, p. 343.
- ^ F. Mosler: Clinical symptoms and therapy of medullary leukemia. In: Berlin Klin Wochenschr . 1876, 13, p. 702.
- ↑ O. Naegeli: Over-red bone marrow and myeloblasts. In: German Med Wochenschr. 1900, 26, p. 287.
- ^ S. Farber, LK Diamond, RD Mercer, RF Sylvester, J. Wolff: Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). In: N Engl J Med. 238, No. 787, 1948, pp. 787-793.