Ionizing radiation

Ionizing radiation (also ionizing radiation ) is a term for any particle or electromagnetic radiation that is able to remove electrons from atoms or molecules (mostly through collision processes) so that positively charged ions or molecular residues remain ( ionization ).
Some ionizing radiation emanates from radioactive substances. The abbreviated term radioactive radiation is sometimes used colloquially for them . Such radiation is also called nuclear radiation .
The designation as ionizing radiation goes back to Joseph John Thomson , who announced on February 27, 1896 that X-rays split the molecules of the air into electrically charged particles and described this as "the air is ionized".
Types of ionizing radiation
Ionizing radiation is any radiation whose kinetic energy (in the case of particles) or quantum energy (in the case of waves) is sufficient to remove electrons from an atom or molecule - also via intermediate reactions. In order to generate the ionization energy required for this , the particle or quantum energy usually has to be more than about 5 electron volts (eV).
- In the electromagnetic spectrum, this corresponds to wavelengths of less than about 250 nm; therefore only have cosmic rays ( cosmic radiation ), gamma radiation , X-radiation and short-wave ultraviolet radiation sufficient to dissolve quantum energy to electrons from the atomic shells and so Covalent bonds separate. In contrast, radio, radar and microwaves, infrared radiation or visible light are not ionizing radiation, because they cannot permanently change or break down molecules (apart from special, light-sensitive substances). Molecules that would be broken down by such low-energy photons could not exist under normal conditions .
- Free protons , electrons or other charged particles are counted as ionizing radiation from a kinetic energy of about 5 eV. Accordingly, alpha radiation (positively charged helium nuclei) and beta radiation (negatively charged electrons or positively charged positrons) are always ionizing radiation.
- Because they are electrically neutral, free neutrons themselves have no noticeable interaction with electrons. However, they ionize indirectly through nuclear reactions or scattering processes on atomic nuclei. The most effective momentum transfer of fast neutrons takes place on hydrogen atom nuclei, which have almost the same mass ( elastic collision ). That is why, on the one hand, water is a good moderator ; on the other hand, fast neutrons are particularly dangerous for living tissue because it always contains water (e.g. in the cytosol ) and its molecules contain hydrogen atoms.
Interaction with matter
Matter shields ionizing radiation through absorption .
The eponymous mechanism - ionization - is the release of electrons from atomic shells. Ionizing radiation is roughly divided into loose and dense ionizing radiation: Radiation from massive particles (protons and ions) is densely ionizing because the particles almost continuously release energy to the penetrated medium on their way and ionize it in the process. Photons (i.e. x-rays or gamma rays ) are thinly ionizing. If there is enough energy transferred to the released electron, one speaks of a delta electron , which in turn can ionize itself. High-energy electrons generate in matter beyond bremsstrahlung , which acts also ionizing. Electron radiation is also counted as loosely ionizing radiation. The paths of ionizing charged radiation particles can be observed in a cloud chamber as traces of fog .
The more densely a type of particle ionizes, the more pronounced the characteristic increase in linear energy transfer / braking capacity , i.e. i. the energy output per distance, towards the end of the path ( Bragg peak ).
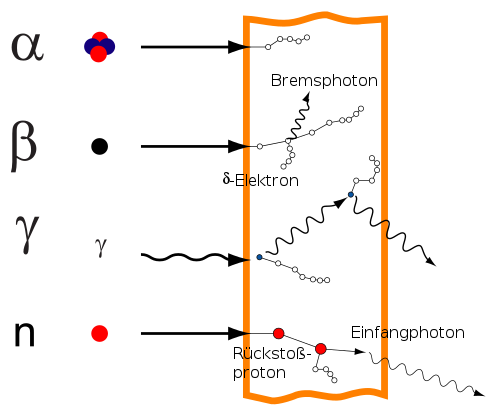
charged particles (e.g. alpha radiation and beta radiation ): directly ionizing uncharged particles (e.g. gamma radiation and neutron radiation ): indirectly ionizing Interaction of ionizing radiation with matter: In the case of the incident neutron, some intermediate processes that are typical in hydrogen-containing material are shown. Gamma quanta are represented by wavy lines, charged particles and neutrons by straight lines or straight lines. The small circles represent ionization processes.
Photons (gamma quanta) do not ionize continuously on their way like alpha or beta particles. The interaction of a gamma quantum with matter occurs through one of the following three processes:
- Photo effect : With the photo effect, the photon knocks an electron out of the shell of an atom.
- Compton effect : With every Compton scattering, the photon gives off energy to a struck electron and flies on in a different direction with reduced energy.
- Pair formation : In pair formation, the photon disappears; its energy leads to the formation of a particle-antiparticle pair.
At low energies and large atomic numbers the photoelectric effect predominates, at high energies and large atomic numbers the pair formation, in between in the range 0.1 to 20 MeV for light elements the Compton scattering (see diagram). If the energy of the photon is sufficiently high, rapid protons or neutrons can also be released through the nuclear photo effect and radionuclides can be formed.
Ionizing radiation breaks up chemical compounds and highly reactive radicals are created . This is where their biologically harmful effect lies. The radiolysis of water is of particular relevance for radiation biology . The reactive oxygen species generated in this way are responsible for the so-called oxygen effect . They react with molecules such as enzymes or DNA , which inactivates or damages them and may have to be repaired . In contrast to thinly ionizing radiation, dense ionizing radiation produces complex DNA damage that is much more difficult to repair, with multiple individual damage in the immediate vicinity, which leads to a higher relative biological effectiveness , which is taken into account in radiation protection by means of higher radiation weighting factors.
Radiation exposure of the population
Natural sources of radiation
The radiation exposure to ionizing radiation from natural sources leads for residents of Germany, depending on the living situation (home, etc.) to an equivalent dose from 1 to 10 mSv per year. These are mainly cosmic rays and radiation from radioactive substances that occur naturally in the earth's crust, building materials and in the atmosphere, e.g. B. the radioactive isotopes of the vital elements carbon and potassium . The human body itself also contains a small amount of these radioactive substances, which is kept constant by the metabolism .
- Naturally occurring radioactivity:
- Radon (can accumulate especially in basement rooms)
- Potassium -40 and other radionuclides in stones and building materials
- Radioactive particles embedded in food
- natural carbon 14 content in food and air
- Cosmic radiation : mainly fast charged particles, secondary radiation through interaction with the atmosphere reaches the earth's surface; responsible z. B. for radiation exposure during air traffic. The load increases with height above sea level.
- Radiation from the sun : Ultraviolet (UV-B is almost completely absorbed, but still leads to sunburn , among other things ; UV-C is completely absorbed in the atmosphere and, by separating the molecular oxygen, leads to the ozone layer), particle radiation ( solar wind ) leads to auroras.
Civilizing radiation sources
The annual dose from civilizing radiation sources is on average in the same order of magnitude as the natural one. She is from
- medical radiation applications such as x-rays or radiation therapy ,
- radioactive material released in previous nuclear weapons tests or nuclear accidents such as Chernobyl ,
- Nuclear reactors and particle accelerators .
X-rays are also inevitably created as a "by-product" in devices in which electrons are accelerated with high voltage, such as tube screens , electron microscopes , radar transmitters or electron beam welding systems . There is a statement on this from the Medical Advisory Board on “Occupational Diseases” at the German Federal Ministry of Labor and Social Affairs.
effect
Sizes and units of measure
Absorbed dose
The absorbed dose is that of an irradiated object, e.g. B. body tissue, amount of energy absorbed per unit mass over a stress period. It depends on the intensity of the irradiation and the absorption capacity of the irradiated substance for the given type and energy of radiation.
Ion dose
The ion dose is a measure of the strength of the ionization, expressed as the charge released per mass of the irradiated substance.
Equivalent dose
The dose equivalent is a measure of the strength of the biological effect of a certain radiation dose; their validity is limited to use in radiation protection . Equivalent doses of the same size are therefore comparable in terms of their effect on humans, regardless of the type and energy of radiation.
The equivalent dose is obtained by multiplying the absorbed dose in gray with the radiation weighting factor (previously called quality factor ), which describes in a simplified way the relative biological effectiveness of the radiation in question. It depends on the type and energy of radiation. For example, the radiation weighting factor for beta and gamma radiation is 1; the equivalent dose in Sv is numerically equal to the absorbed dose in Gy. For other types of radiation, factors of up to 20 apply (see table in radiation weighting factor).
See also: Order of magnitude (dose equivalent)
Biological effect
Radicals generated by ionizing radiation usually cause greater damage from subsequent chemical reactions than the destruction of the first molecule by radiation alone. This effect is desirable , for example in the fight against cancer , as it favors the death of affected cells, in this case ideally tumor cells. The Radonbalneologie sets of the noble gas on the therapeutic effect of radon in certain diseases.
Opinions differ on the extent of the harmfulness:
- Radiation sickness occurs from short-term exposure of around 0.2 to 1.0 Sv . 4 Sv as short-term irradiation are fatal in 50% of cases, 7 Sv are definitely fatal. It manifests itself in a weakened immune system and burns . Without a doubt, from a high radiation dose (greater than about 2 Sv ) so many molecules with a biological function are destroyed at once that the affected cells are no longer viable. Too many toxic substances are also created from the breakdown of molecules that kill the cell. At the molecular level, among other things, the harmful effects of radicals caused by radiolysis are involved. As a long-term consequence, changes in the genetic material are also frequent, which with a certain probability can lead to cancer , but above all to mutations that can lead to malformations in offspring or developing embryos / fetuses as well as total sterility (infertility) (see also radiation risk ).
- At mean life doses of around 0.1 Sv , which roughly corresponds to the dose that a person ingests over the course of 76 years due to the constant natural radiation of (in Germany) up to 1.3 mSv / a, there are no striking observations. because apparently all living beings have adjusted to it in the course of evolution.
- The effects of very low doses around 0.02 Sv are controversial:
- Some experts suggest that the harmfulness of ionizing radiation decreases linearly with decreasing dose. Since the risk of dying from cancer is only increased by 1 ‰ at 0.02 Sv according to the linear model, millions of test persons would be required for reliable statistical evidence. Such proof is not possible.
- Significantly fewer scientists are registering indications that lower radiation exposure can also cause greater damage; for example because the immune system “falls asleep” due to a lack of activity and the susceptibility to disease increases. It is controversial whether a reduction in natural radiation exposure can promote disease (see Hormesis ).
The alpha radiation has on living tissue by their ionizing power particularly high harmful effect, but it has to air a range of only a few centimeters and can by a simple sheet of paper completely shielded be (the same purpose meet the top dead skin flakes), so that alpha emitters that are located outside the human body are largely harmless. Alpha emitters are dangerous when they come into direct contact with living tissue. One way of doing this is to inhale aerosols that are absorbed through the mucous membranes of the airways; radioactive dust is stored in the lungs and can cause cancer there. Due to its chemical properties, the noble gas radon is not stored in the body, but it is endangered by radioactive decay in the lungs during inhalation . If a very powerful alpha emitter (half-life of a few days or less) has been ingested through food or injected into the bloodstream, even a few micrograms can be fatal to humans.
Also, ultraviolet radiation can act ionizing because the shorter wavelength components, due to the ozone layer only reach a small proportion of the sun on the earth's surface, increase the risk of skin cancer.
Other effects
Ionizing radiation can cause errors in microelectronic circuits (chips) (bit errors in RAM etc.). These errors occur all the more frequently, the lower the charges on the respective components. They are therefore the most disturbing in very small structures. The stability against such errors is an important design criterion. Suitable protective measures must be taken, especially for use in space.
With EPROMs , the ionizing effect of ultraviolet light is used specifically for erasure.
Biological and chemical applications of ionizing radiation
In biology mainly the mutating and sterilizing effect is used. In plant breeding, for example, "radiation-induced mutations" ( mutagenesis ) are generated, which can produce modified species . One field of application is the “ sterile insect technology ”, SIT for short. Male insect pests are sterilized by gamma radiation and then released in the target area. The absence of offspring leads to a decrease in the population. The advantage here is that no harmful chemicals are used and other insects remain unaffected.
Ionizing radiation is also suitable for the sterilization of devices, implants, food and drinking water. This kills microorganisms. However, strict requirements apply to the radiation sterilization of food. The growth of a seedling can be improved by weak radiation, whereas excessive radiation has a growth-inhibiting effect.
In the production of polymers , irradiation allows crosslinking without generating heat. Large components can also be networked with radiation that penetrates far. Beta radiation (radiation-crosslinked insulating materials) and ultraviolet radiation (hardening of synthetic resin paint layers) are used, among other things. When activators are added, some polymer reactions can also be initiated by irradiation with visible light.
Ionizing radiation can cause color changes in gemstones, glasses and pigmented plastics. In crystals like corundum , this is done by creating color centers .
The photolithography (u a in the.. Microelectronics - and printed circuit board manufacturing ) uses crosslinking reactions (positive resist) or decomposition reactions (negative resist) which are caused by ultraviolet, X-ray, ion or beta radiation.
Ultraviolet radiation can be used for chlorine-free bleaching of cellulose . The coloring (dirt) components of the fabrics are chemically split up and converted into volatile or washable substances.
Radiation protection
Humans cannot perceive ionizing radiation directly, whether from radioactive or other sources. Special care is therefore required for effective radiation protection when handling radioactive materials. Shielding, maintaining a large distance and restricting the length of stay in the radiation field ( 3-A rule ), if necessary the use of measuring and warning devices ( dosimeters ) are helpful .
literature
- Hanno Krieger: Basics of radiation physics and radiation protection . 4th edition, Springer 2012, ISBN 978-3-8348-1815-7 .
Web links
- The "Glossary radiation protection" of the Jülich Research Center explained many terms round (etc. units, dose terms, alpha, beta, gamma radiation, radiation protection) to ionizing radiation.
Individual evidence
- ^ Heinz Otremba: Wilhelm Conrad Röntgen. A life in the service of science. A documentation with a scientific appreciation by Walther Gerlach . Franconian company printing house, Würzburg 1970, p. 55.
- ^ Radiation Oncology Physics Handbook IAEA, Division of Human Health, Dosimetry and Medical Radiation Physics. Chapter 19, p. 487. Retrieved March 2, 2015.
- ↑ Health risks from exposure to low levels of ionizing radiation Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Board on Radiation Effects, Research Division on Earth and Life Studies, National Research Council of the National Academies. ISBN 0-309-09156-X (paperback), ISBN 0-309-53040-7 (pdf). P. 19. Accessed March 2, 2015.
- ↑ Eric J. Hall, Amato J. Garcia: Radiobiology for the Radiologist, 7th Edition, Lippincott Williams & Wilkins 2012, ISBN 978-1-4511-5418-4 .
- ↑ Scientific opinion on diseases caused by ionizing radiation
- ↑ Ray F. Evert, Susan E. Eichhorn: Esaus plant anatomy meristems, cells and tissues of plants - their structure, function and development . Walter de Gruyter, 2009, ISBN 978-3-11-020592-3 , p. 108 ( limited preview in Google Book search).
- ↑ Claus Grupen, Tilo Stroh, Ulrich Werthenbach: Basic course on radiation protection, practical knowledge for dealing with radioactive substances . Springer-Verlag, 2008, ISBN 978-3-540-75849-5 , pp. 191 ( limited preview in Google Book search).
- ↑ Heinz M. Hiersig : Lexikon production technology process technology . Springer-Verlag, 2013, ISBN 978-3-642-57851-9 , pp. 85 ( limited preview in Google Book search).
- ↑ Werner Stolz: Radioactivity Basics - Measurement - Applications . Springer-Verlag, 2013, ISBN 978-3-663-01497-3 , pp. 166 ( limited preview in Google Book search).
- ^ Hans J. Mair: plastics in cable technology development, testing, experience, tendencies; with 34 tables . expert verlag, 1999, ISBN 978-3-8169-1511-9 , p. 279 ( limited preview in Google Book search).
- ↑ Bodo Müller, Johann Georg Leutmann, Ulrich Poth: Paint formulation and paint recipe, the textbook for training and practice . Vincentz Network GmbH & Co KG, 1978, ISBN 978-3-87870-170-5 , p. 239 ( limited preview in Google Book search).
- ↑ Florian Neukirchen: Gemstones Brilliant witnesses for the exploration of the earth . Springer-Verlag, 2012, ISBN 978-3-8274-2922-3 , pp. 9 ( limited preview in Google Book search).
- ↑ Andreas Risse: Manufacturing processes in mechatronics, precision engineering and precision device technology . Springer-Verlag, 2012, ISBN 978-3-8348-8312-4 , pp. 524 ( limited preview in Google Book search).