Radon decay products
Radon decay products arise from the radioactive noble gas radon through nuclear decay . The air we breathe outdoors, in apartments and especially in burrows, contains a mixture of radon and its decay products. Radon is responsible for around 10% of all lung cancers. The cause is its short-lived decay products.
Radiation effect
A few hundred years ago, especially in the mining area of Schneeberg and Joachimstal, most of the miners died of Schneeberg disease , which was later identified as lung cancer . At the beginning of the 20th century, the high radon content of the air in these mines was noticed and suggested a connection with the disease. It was not until the 1950s that radiation protectionists realized that inhaling the radon decay products leads to a high dose of alpha radiation in the bronchial epithelium and thus to lung cancer. Professional radiation protection in uranium and other mines began.
The isotopes radon-222 and radon-220 are of radiological importance . During inhalation, their decay products are deposited in the respiratory tract and accumulate there. Only the short-lived isotopes of the respective decay series are important . The organism secretes the long-lived isotopes that are also present, so that their radiation is hardly effective. The most biologically effective alpha radiation comes from the polonium isotopes among the decay products.
In common parlance, radon refers to radon-222 including its decay products. Limit values for radon gas always include the effect of the decay products. If only radon gas or the decay products are meant, this is usually expressly pointed out. Radon-220 also has the historical name Thoron . Radon and thoron often occur together. The radiation dose from thoron is usually 10 times lower than that from radon.
Decay series from the point of view of radiation protection
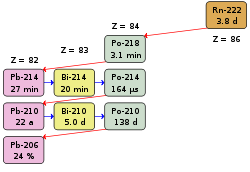
222 radon decay series
Radon-222 breaks down one after the other into the nuclides shown in the table . This is the simplified last part of the uranium-radium series . The most important figures for radiation protection are given in bold. The data in the last three columns are useful for calculating the Potential Alpha Energy (PAE) (see below).
HWZ : half-life (d days a year)
PAE / atom : Potential alpha energy per atom
atoms / Bq : number of atoms per unit of activity Becquerel
PAE / Bq : Potential alpha energy per unit of activity Becquerel
| nuclide | Decay | HWZ | α energy | PAE / atom | Atoms / Bq | PAE / Bq |
| Rn-222 | α | 3.825 d | 5.49 MeV | 0 | 0 | 0 |
| Po-218 | α | 3.05 min | 6.00 MeV | 13.68 MeV | 264 | 3612 MeV |
| Pb-214 | β | 26.8 min | 7.68 MeV | 2320 | 17820 MeV | |
| Bi-214 | β | 19.9 min | 7.68 MeV | 1710 | 13130 MeV | |
| Po-214 | α | 0.164 ms | 7.69 MeV | 7.68 MeV | 0.000231 | 1.77 keV |
| Pb-210 | β | 22.3 a | 0 | |||
| Bi-210 | β | 5.01 d | 0 | |||
| Po-210 | α | 138.4 d | 5.30 MeV | 0 | ||
| Pb-206 | stable | 0 |
220 Radon Decay Series
Radon-220 breaks down one after the other into the nuclides listed in the table . This is the simplified last part of the Thorium series . The most important figures for radiation protection are given in bold. The data in the last three columns are useful for calculating the Potential Alpha Energy (PAE) (see below).
HWZ : Half-life (d days a year)
PAE / atom : Potential alpha energy per atom
atoms / Bq : number of atoms per unit of activity Becquerel
PAE / Bq : Potential alpha energy per unit of activity Becquerel
| nuclide | Decay | HWZ | α energy | PAE / atom | Atoms / Bq | PAE / Bq |
| Rn-220 | α | 55 s | 6.29 MeV | 0 | 80.2 | 0 |
| Po-216 | α | 0.15 s | 6.78 MeV | 14.61 MeV | 0.216 | 3.16 MeV |
| Pb-212 | β | 10.64 h | 7.83 MeV | 55.053 | 431,065 MeV | |
| Bi-212 | 35% α 65% β | 60.6 min | 6.07 MeV | 7.83 MeV | 5,246 | 41,076 MeV |
| Po-212 | α | 304 ns | 8.78 MeV | 8.78 MeV | 0.44 e-06 | 3.85 eV |
| Tl-208 | β | 3.05 min | 0 | |||
| Pb-208 | stable | 0 |
The PAE / atom of the Bi-212 is calculated according to the branch probabilities in Tl-208 and Po-212:
- 0.35 x 6.07 MeV + 0.65 x 8.78 MeV = 7.83 MeV
Binding to aerosol particles
In the air, individual decay product atoms are created by nuclear transformation from the radon atoms present there or other decay product atoms that have already formed. If a decay product atom hits an obstacle, it attaches itself to it and stays there. Obstacles are mostly aerosol particles in the air, but also walls or furniture. Because individual decay product atoms move very quickly in the air by diffusion and there are usually many dust or aerosol particles, the decay product atoms hit a particle very quickly and stick to it. It is then an accumulated product of decay , and the aerosol particles determine its further behavior. This includes movement in the air and deposition on objects and in the respiratory tract.
In contrast to inactive heavy metal atoms, radioactive decay product atoms can detach themselves from a dust particle. This happens in the nuclear transformation through the recoil when a radiation particle is emitted.
Decomposition products that are not attached to aerosol particles are called free decay products . The proportion of free decomposition products is 1% or less in normal air. However, it can be much greater in particularly clean air or with freshly added radon gas.
The separation probability of aerosol particles and deposited decay products in the respiratory tract is approximately 10%. The person breathes out the rest again. Free decay products are 100% separated in the respiratory tract due to their rapid diffusion. Therefore, free decay products are more dangerous than deposited ones. Compared to breathing air without free decay products, for example, air with 10% free decay products generates approximately twice the inhalation dose.
Some filter devices promise a reduction in the radiation dose in lounges because they remove the harmful decomposition products from the air we breathe. However, they also remove the aerosol particles. First of all, low-dust, decomposition-product-free air flows out of the filter device. Because the radon gas is still present, new decay products form quickly. This creates a mixture with less radioactivity but a higher proportion of free decay products. As a result, the radiation dose is usually only slightly reduced. Under unfavorable conditions, a filter device can even increase the radiation dose.
Occupational radiation protection uses work helmets with built-in fans and filters. The procedure works here because the helmet blows filtered air directly in front of the face. Hardly any decay products are formed in the short time available.
Potential alpha energy concentration
Concept of radiation protection for radon inhalation
The concentration of radon decay products in the form of the potential alpha energy concentration (PAEK) in the air we breathe is a measure of the harmful effect of a mixture of the radioactive noble gas radon and its radioactive decay products. Occupational radiation protection regulations limit the PAEK. One advantage of this concept is that only a numerical value is required to assess the breathing air and not the activities of all decay products that occur.
The radon gas concentration is usually taken into account if inexpensive measurement methods are used, which can only record the concentration of the radon gas and not that of the decay products. Statutory or recommended limit values for radon gas basically evaluate the decay products that occur together with it.
In connection with radon inhalation, radiation protection only takes into account the alpha rays of all atoms that can be deposited in the respiratory tract. This also includes atoms that are not alpha emitters themselves when they are later transformed into alpha emitters. Radon gas is not one of them because most of it is exhaled again. The remaining gas component in the body leaves the respiratory tract very quickly and is distributed throughout the entire organism via the blood. Also, only the short-lived decay products with half-lives of up to a few hours are taken into account, because the organism excretes long-lived ones before a significant radiation effect can occur.
Potential alpha energy of an atom
If a person inhales a decay product atom, the alpha energy that will later be released at some point is the quantity relevant in radiation protection. This is called Potential Alpha Energy (PAE). Each decay product atom is assigned the PAE that it will emit by the end of the short-lived part of the radon decay series. Tables 1 and 2 contain the PAE of all possible decay product atoms (PAE / atom).
Potential alpha energy in air
The potential alpha energy contained in a volume of air is the sum of the PAE of all atoms that are in this volume. The radioactivity can be given as a list of the activities of all radionuclides present. From these activities and the half-lives of the nuclides, the law of decay can be used to calculate how many atoms are present for each nuclide. Tables 1 and 2 contain the numbers of atoms in each case for the unit of activity 1 Bq.
The potential alpha energy concentration can be calculated immediately using the table information if the activity concentrations of all short-lived decay products are known. In practice it is not necessary to first determine all individual activity concentrations in order to calculate the PAEK from them. There are also methods that can measure the PAEK with sufficient accuracy without this detour.
Equilibrium factor
If the only source and sink for the activity of radon or thoron and their decay products is radioactive decay, i. H. if other sources or sinks such as ventilation , deposition or filtering of the air are negligible, a radioactive equilibrium is established between the nuclides. The sum of the potential alpha energy concentrations of all secondary products is 5.5 nJ per Bq radon and 75 nJ per Bq thoron for the respective secondary products; for radon, only the short-lived secondary products relevant to radiation protection are considered here. The ratio of the PAEK of the secondary products actually present in a sample to this value is referred to as the equilibrium factor F. If sinks for secondary products predominate, for example in the case of air samples in the form of deposition on surfaces, F <1, while sources predominate, which is rare, is F > 1.
literature
- GSF - Research Center for Environment and Health : Radiation in Everyday Life . Munich 1991, ISSN 0175-4521