Stem cell transplant
Under stem cell transplantation is the transfer of stem cells from a donor to a recipient. The donor and recipient can be the same person ( autologous transplant ) or two different people ( allogeneic transplant).
In clinical practice today only blood stem cells are transplanted. Blood stem cells are also known as hematopoietic stem cells; hence the abbreviation HSZT (for hematopoietic stem cell transplantation), based on English. Other, non-haematopoietic stem cells , such as mesenchymal stem cells , have already been transferred in clinical studies . However, these transplants are currently not of practical importance for medicine (see regenerative medicine , stem cell therapy, etc.). In the following, therefore, only the transplantation of blood stem cells is mentioned. There are three different types of transplantation: allogeneic, autologous and syngeneic. The allogeneic is the most common type of transplant, in which people with compatible tissue characteristics are sought worldwide. In the case of autologous transplantation, stem cells are taken from the patient himself and these are then returned to the patient after appropriate myeloablative (i.e. bone marrow-destroying) therapy. The syngeneic is the rarest type of transplant because you have to find a twin of the recipient.
Indication for a stem cell transplant
An autologous transplant may be necessary if e.g. As due to cancer a chemotherapy and / or radiation is necessary. However, since these can seriously damage the own blood-forming system, healthy stem cells are removed from the patient before treatment begins, and these are returned to him after the myeloablative (bone marrow-eliminating) therapy. The bone marrow function can thus be restored. The autologous stem cell transplant introduced in the 1970s made high-dose chemotherapy, e.g. B. in neuroblastoma , and thus significantly increased the chances of recovery, especially in childhood malignant tumors. Another application is the removal of diseased stem cells for treatment outside the patient's body.
Allogeneic stem cell transplants are mainly used for the various forms of leukemia , when other treatment methods have not been successful, but also for various other diseases such as B. Malignant lymphoma . Often times, a transplant is the only way the patient can fully cure. In Hodgkin's lymphoma, however, the benefit of allogeneic stem cell transplantation has not been proven.
The first successful allogeneic stem cell transplants were carried out in 1968 on patients with the inherited immunodeficiency diseases X-SCID and Wiskott-Aldrich syndrome .
Requirements for a successful transplant
The most important requirement for a successful (allogeneic) transplant is the availability of a compatible donor. For this purpose, certain tissue characteristics, the so-called HLA types, are examined. Since the tissue characteristics allow millions of combinations due to their diversity, the search for the right donor turns out to be extremely difficult. The more precisely the donor and recipient match in their HLA characteristics, the greater the likelihood of a successful transplant. Conversely, with every discrepancy in the HLA characteristics, the so-called HLA mismatches , the chance of successful growth of the transplanted cells decreases and the risk for the patient increases. For example, if the characteristics do not completely match, the likelihood of developing a so-called graft-versus-host (GvH) reaction increases, a rejection reaction in which the transplanted immune cells of the donor recognize the patient's organs as foreign and fight them. Although the effects of GvH can usually be controlled with medication, so that recipients can feel slight (sometimes chronic) discomfort, more severe GvH courses can cause organ damage or lead to the death of the recipient. Mesenchymal stromal cells could be used for the therapeutic and prophylactic treatment of GvH responses. In 2019, Fisher and coworkers created a Cochrane review of randomized controlled trials to measure the safety and efficacy of mesenchymal stromal cells (MSC) in patients with a graft-versus-host reaction (GvHD). The evidence is very uncertain about the effect of mesenchymal stromal cells in the treatment of graft-versus-host diseases after stem cell transplants on the complete remission (= complete disappearance) of acute and chronic graft-versus-host reactions when used therapeutically. Mesenchymal stromal cells may cause little or no change in all-cause mortality, return of malignancy, and the incidence of acute and chronic graft-versus-host reaction for prophylactic purposes.
When searching for a donor, the ( AB0 ) blood group does not play an essential role - the donor and recipient can, under certain conditions, have different blood groups. Since the recipient's entire diseased hematopoietic system is destroyed before the transplant and is replaced by the donor's stem cells, the recipient always has the donor's blood group after a successful transplant - even if he had a different blood group before.
In most cases, close relatives (siblings) are most likely to be considered as donors. However, there is no suitable family donor available for a large number of patients - for this case there have been databases for some years in which the HLA characteristics of many millions of voluntary donors are stored. Nevertheless, the search for a suitable third-party donor usually takes a few months.
Donor search and donor register
Only about 30% of all patients who need a stem cell donation from someone else can find a suitable donor among their own relatives. The rest are dependent on an external donor. Numerous organizations around the world that operate a bone marrow donor database have been set up to find such donors .
Interested donors can have themselves typed at one of these organizations or partially in pharmacies, the easiest way to do this at a typing campaign carried out on site . A small blood sample (about 2–20 ml, which corresponds to a maximum of about two tablespoons ) is taken or a smear is taken from the inner wall of the cheek, with the help of which the most important tissue characteristics can be determined in the laboratory. The swab of the tissue can be done at home and sent by mail. These are stored by the respective organization together with the name and address of the potential donor. The personal data are only used to be able to contact the donor later. All information is only entered in the national and international databases in anonymised form under a donor code. With the inclusion in a donor register, however, nobody undertakes to actually donate later.
So far, around 6.5 million willing donors have been registered and HLA- typed in Germany (as of February 14, 2016), worldwide there are a total of 27.7 million (as of January 27, 2016).
If a patient needs a third-party donation, the treating physicians turn to so-called search centers , which are usually affiliated to large transplant clinics or donor files, to initiate a donor search; in Germany this is usually the Central Bone Marrow Donor Register Germany . These registers look for suitable donors in the international databases. The aim is to find a donor that is as HLA-compatible as possible. However, other factors also play a role, such as the age, weight and gender of the potential donor, as the available number of stem cells required for a possible transplant is also influenced by these factors. Eligible donors are then asked via their donor files to have their blood drawn again so that compatibility with the patient can be determined more precisely ( fine or high resolution typing ).
With the help of international donation registers it is now possible to find this genetic twin for around 70% of those in need who do not already have a suitable donor in their families.
However, it has a negative impact on the donor search that some donors do not inform their donation register when they move and they can no longer be found (e.g. due to the lack of registration laws , such as in the USA ). It has already happened that, despite very rare tissue characteristics, a suitable donor was already in the databases, but could no longer be located due to a move.
If a suitable donor is finally found, its tissue characteristics are tested again (so-called confirmation test or confirmatory typing ) and the ability to donate is assessed using a medical questionnaire and virological examinations . This is done in order to be able to exclude all risk factors that could later endanger the donor or the recipient. The donor files are required to the donor via abnormal findings (eg positive findings for. Hepatitis - or HIV - markers or rare blood characteristics) to be informed.
The donor can withdraw from the donation at any time up to the beginning of the pretreatment of the patient (see below). If possible, this should be avoided at such a late point in time, as a lot of time and money has already been invested in the search for donors by then. Stepping back shortly before the actual donation (i.e. when the patient has already been pretreated) leads to the death of the patient in most cases, since the pretreatment is often associated with significant complications that the body can rarely cope with without the transplant.
In principle, donors are reimbursed for all necessary expenses from the time they are asked to do a new blood test. This includes the travel costs to the preliminary examinations and the donation itself as well as the loss of earnings for these days. In the case of employees, wages are usually continued to be paid, and the employer can invoice the respective donor center for this amount. In addition, most of the files take out insurance for the donors, which covers incidents during the preliminary examinations and the actual collection, as well as accidents during trips to these appointments.
During the entire donation process, neither the recipient nor their doctors learn the identity of the donor, nor does the donor know who the patient is (of course, this does not apply to donations between family members). About six to eight weeks after the transplant, the donor can use his donor database to find out about the recipient's state of health. Since the transplant takes some time to grow, it is not possible to give reliable information beforehand. But even after this period of time, it is difficult to make definitive statements, as the diseases can relapse even after a successful transplant. But if everything went well, contact with the patient or the donor is possible in many cases. Depending on the guidelines of the respective donor or patient registry , the donor and his recipient can either send anonymous letters via the donor database or the transplant center after a certain period of time or get to know each other personally after a certain period of time, if both so wish. In German patients, the time between the last transplant and getting to know each other is two years. But there are also countries in which getting to know each other in this way is fundamentally not possible.
Methods of stem cell extraction
Basically there are currently two methods of stem cell collection, the classic bone marrow collection and the now more common peripheral blood stem cell donation . With both methods one achieves qualitatively equivalent results. The donor is therefore fundamentally free to choose which of the two options he or she is ready for.
In addition, there is the possibility, with certain restrictions, of obtaining stem cells ( umbilical cord blood stem cells ) from umbilical cord blood .
The transplant does not necessarily have to be removed in the clinic where the patient is being treated; rather, the donor files try to organize a collection near the donor's place of residence so that he does not have to travel far. The transplant is brought to the patient on the same day by a courier (usually the patient's doctor or an employee of the clinic).
Bone marrow donation
The classic method of stem cell transplantation is the transfer of red bone marrow . As a rule, about 1 liter of bone marrow-blood mixture is removed from the donor from the iliac crest using a special needle. The stem cells are isolated from this and, if necessary, further purified and later transfused to the recipient .
The removal procedure takes place under anesthesia and takes about 1 to 1½ hours, including induction and discharge of the anesthesia. Although outpatient removals are also possible, the donor is usually admitted to the hospital the evening before the removal and discharged the day after the removal in order to have another night of control after anesthesia and removal. The removed bone marrow regenerates from the donor within about two weeks; Iron tablets are often given home to support blood formation .
Peripheral blood stem cell donation
In the meantime, the classic bone marrow donation has largely been replaced by the peripheral blood stem cell donation . The donor is injected with the hormone G-CSF for about a week , which causes stem cells to pass from the bone marrow into the blood. There they can then be filtered out using stem cell apheresis (for a more detailed description, see there).
Cord blood donation
A special form of stem cell transplant is the use of umbilical cord blood . Immediately after the birth, the blood from the clamped umbilical cord is sucked off (otherwise it would be thrown away) and the stem cells extracted in the laboratory. The stem cells obtained in this way are particularly well tolerated, but are naturally only available in smaller quantities. Parents can donate their children's umbilical cord blood or have it frozen for their own use for a fee.
Risks to the donor
As with any medical intervention, a stem cell transplant is associated with possible risks and side effects for the donor, which can be caused by the invasive intervention and the medication administered. A comprehensive health check is carried out before every stem cell donation, which keeps the possible risks of the transplant as low as possible. Reasons for exclusion are e.g. B. an age over 61 years, cardiovascular diseases, autoimmune diseases, diseases of the kidney and infectious diseases. Mental illness and the presence of an addictive illness also usually lead to exclusion. This ensures that only healthy and resilient donors are used and that potential risk factors can be excluded in advance.
In general, stem cell transplantation is considered a relatively low-risk procedure for the donor. Nevertheless, complications can arise during the implementation, and in rare cases severe and permanent damage is documented. In very rare individual cases, deaths after stem cell donation have also been reported.
Bone marrow donation
The possible side effects for the bone marrow donor are usually limited to slight pain and restricted mobility (similar to sore muscles ) as well as bruises in the area of the puncture sites, which however disappear again after a few days. The necessary anesthesia can also lead to temporary nausea or the like. According to the US Department of Health, the risk of serious complications from anesthesia or puncturing the bone marrow space is around 2.4%. However, 99% of all donors fully recover. According to one study, the participating European transplant teams had a single death between 1993 and 2005, out of a total of 27,770 bone marrow donations. Compared to the normal population, no increased risk of cancer was found in a period of several years after the operation.
The term bone marrow is often confused with spinal cord in connection with a stem cell transplant . However, this is wrong; no surgery is performed on the spine when bone marrow is removed. Limitations in sensibility or even paraplegia are therefore generally not to be feared.
Peripheral blood stem cell donation
As side effects, the donor often experiences more or less pronounced flu-like symptoms, which are caused by the medication administered (e.g. filgrastim) and which usually disappear again quickly after it is discontinued. According to a study of 2408 donors, 40% of patients report tiredness, headaches and insomnia after the procedure. These symptoms usually go away over the course of a month. Perceived bone pain subsides within a week in 94% of patients. However, up to 2% of the patients were not completely pain-free even one year after the procedure. Through the simultaneous administration of common, over-the-counter pain relievers such as B. Paracetamol or ibuprofen can often greatly alleviate these symptoms. About two-thirds of patients with moderate to severe pain respond to these pain medications. 3% of the donors reported permanent fatigue that persisted for a long time after the procedure. Fifteen of 2408 donors (0.6%) had severe complications that required a long hospital stay. However, no deaths occurred in this study. The cancer rates of the donors were also not increased in a period of 8 years after the operation compared to the normal population.
According to a survey of 262 stem cell transplant teams in European specialist clinics, the risk of serious cardiovascular problems after the procedure is around 1: 1500. In this study, 4 deaths were reported out of a total of 23,254 performed peripheral blood stem cell donations. Here, too, the number of cancer cases after the operation was not increased compared to the normal population over a period of several years.
Cord blood donation
Clamping and severing the umbilical cord after birth is a necessary and natural process in the birth of a child. The blood contained in the umbilical cord, if not donated, would be sucked off and disposed of unused. Since no additional medical intervention is necessary during the normal birth process, umbilical cord blood donation is therefore risk-free for mother and child according to the current state of knowledge.
Procedure of the transplant at the recipient
Basically, the stem cells obtained are transferred to the patient intravenously . To do this, however, the patient's own diseased bone marrow must first be destroyed with radiation and / or chemotherapy . This phase of preparation is called conditioning . The more thoroughly this is done, the more severe the side effects, but the lower the risk of relapse. The treating doctors make the decision on the intensity of the pre-treatment. The remnants of the old bone marrow are then destroyed by the new immune system that came from the donor. For this reason, identical twin siblings are not necessarily the ideal donors: although the compatibility of the transferred stem cells is particularly good here, the remains of the diseased bone marrow may not be completely eliminated. The same applies to autologous transplants.
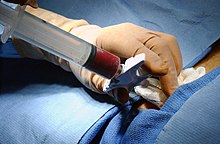
The actual transplant is inexpensive: the transplant is transferred directly from the bag (see picture) through a venous catheter into the recipient's bloodstream. The new bone marrow finds its way into the bones by itself and starts producing blood cells after about ten days.
After the transplant, the patient is exposed to an increased risk of infection . This is due on the one hand to the necessary immunosuppression and on the other hand to the fact that the donor's immune system is transferred to the new body, but not the information about diseases that have already been overcome. The patient's immune system is more or less the same as that of an infant, and in fact many stem cell recipients subsequently develop typical childhood diseases, even if they have already had them. Only after a few years do the body's defenses return to those of a healthy adult.
A stem cell transplant costs around 98,000 euros in Germany (as of 2012).
Stem cell transplant with reduced conditioning
In classic allogeneic SCT, several drugs are used in high doses during conditioning, usually combined with whole-body irradiation, to eradicate all leukemic or malignant cells and suppress the immune system. Associated with this is a destruction of the bone marrow. This is why this type of SCT is also referred to as myeloablative (destroys bone marrow).
Stem cell transplantation with reduced conditioning intensity (RIC), on the other hand, essentially only aims at immunosuppression with appropriate immunosuppressants in order to switch off the immune system before the transplantation so that the risk of non-growth or rejection of the transplanted donor stem cells is kept as low as possible. This type of transplant is less aggressive. After the donor stem cells have grown, they should then be able to destroy any leukemic cells that are still present with the help of an immunological reaction, the so-called transplant counter-leukemia reaction. The advantage of the RIC-SCT is that it can also be used to cure older patients and patients with advanced or difficult-to-cure leukemic diseases. Since this procedure is still relatively new, the significance of this type of transfer in comparison to classic stem cell transplantation cannot yet be precisely determined. Life-threatening complications, such as with classic SCT, often occur with the same intensity. In particular, these are severe infections and the graft-versus-host reaction ( GvHD ), which occurs relatively late after the transplant and can also develop chronically.
In a 2011 study by the Fred Hutchinson Cancer Center in Seattle (USA), the treatment outcome was examined in elderly patients with advanced hematological cancers who received an allogeneic stem cell transplant with reduced conditioning. From 1998 to 2008, 372 patients aged 60–75 years (average age 64 years) from 18 clinics were recorded. The five-year survival rate was about a third of the patients. Disease progression or relapse was the leading cause of death in 135 patients (36%). 104 patients (28%) died from other causes, such as infection, donor-versus-host reaction (GvHD) and multiple organ failure.
According to a German study from 2012, 195 patients suffering from AML were either intensively pretreated or with a low dose. The survival rate of the patients after three years remained almost the same, it was around 60 percent in both groups. However, there were clear differences in the early phase: With reduced conditioning, only 8 percent of the patients died one year after the start of treatment, but 17 percent in the scheme with high-dose pretreatment. The age of the recorded patients was between 18 and 60 years.
Research with a broad impact
Stem cell research in Germany also aims to improve transplants in older cancer patients. In 2011, the German Cancer Aid funded seven research projects of a priority program with 3.2 million euros, so that the therapy of cancer patients in older age becomes more effective.
See also
- Central Bone Marrow Donor Register Germany
- German bone marrow donor center
- Bone marrow donation campaign in Bavaria
- Stefan Morsch Foundation
- German José Carreras Leukemia Foundation
- German stem cell donor database
- Chernobyl nuclear disaster # Other countries
literature
- Susanne Schäfer : siblings of humanity . Experience report from a bone marrow donor. Glaré-Verlag, Frankfurt 2009. ISBN 978-3-930761-69-2 .
- Paul Paulson In Vivo - Diagnosis Lymph Node Cancer: Researched and Experienced , Prof. Dr. Michael Hummel co-author on the oncological background. Epubli Verlag, 2012. ISBN 978-3-8442-1843-5
Web links
Sources and further information on stem cell donation and transplantation:
- Central Bone Marrow Donor Register Germany (central, anonymised register of all German donors)
- German stem cell donor database DSD
- Austrian red cross
- Austrian stem cell register
- Swiss Blood Stem Cells Foundation
- German José Carreras Leukemia Foundation V.
- zellux.net: Comprehensive themed portal with teaching material on the topic of stem cell research
- DLH: stem cell transplant with reduced conditioning
Individual evidence
- ↑ Deutsches Ärzteblatt, September 28, 2010: Benefits of allogeneic stem cell transplantation at Hodgkin not proven ( memento of the original from April 21, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Sheila A Fisher, Antony Cutler, Carolyn Doree, Susan J Brunskill, Simon J Stanworth: Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition . In: Cochrane Database of Systematic Reviews . January 30, 2019, doi : 10.1002 / 14651858.CD009768.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ Carolin Bauer: Pharmacies mediate stem cell donors. Retrieved July 11, 2016 .
- ↑ current donor numbers ZKRD
- ↑ About the ZKRD
- ↑ German standards for unrelated blood stem cell donation, here Section 3.3 (.9) ( Memento of the original from January 21, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Central Bone Marrow Donor Register Germany: Risks of a Donation , accessed on September 2, 2017.
- ↑ WSZE: Reasons for exclusion for stem cell donations , accessed on September 2, 2017.
- ↑ D. Pamphilon, S. Siddiq, S. Brunskill, C. Dorée, C. Hyde, M. Horowitz, S. Stanworth: Stem cell donation - what advice can be given to the donor? In: British Journal of Hematology . Volume 147, number 1, October 2009, pp. 71-76, doi : 10.1111 / j.1365-2141.2009.07832.x , PMID 19681886 , PMC 3409390 (free full text) (review).
- ↑ FAQ of the US Department of Health on Bone Marrow Donation , accessed September 23, 2017.
- ^ Donor safety and support , accessed September 23, 2017.
- ↑ J. Halter, Y. Kodera, AU Ispizua, HT Greinix, N. Schmitz, G. Favre, H. Baldomero, D. Niederwieser, JF Apperley, A. Gratwohl: Severe events in donors after allogeneic hematopoietic stem cell donation. In: Haematologica. Volume 94, number 1, January 2009, pp. 94-101, doi : 10.3324 / haematol.13668 , PMID 19059940 , PMC 2625420 (free full text).
- ↑ MA Pulsipher, P. Chitphakdithai, JP Miller, BR Logan, RJ King, JD Rizzo, SF Leitman, P. Anderlini, MD Haagenson, S. Kurian, JP Klein, MM Horowitz, DL Confer: Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. In: Blood. Volume 113, number 15, April 2009, pp. 3604-3611, doi : 10.1182 / blood-2008-08-175323 , PMID 19190248 , PMC 2668845 (free full text).
- ↑ J. Halter, Y. Kodera, AU Ispizua, HT Greinix, N. Schmitz, G. Favre, H. Baldomero, D. Niederwieser, JF Apperley, A. Gratwohl: Severe events in donors after allogeneic hematopoietic stem cell donation. In: Haematologica. Volume 94, number 1, January 2009, pp. 94-101, doi : 10.3324 / haematol.13668 , PMID 19059940 , PMC 2625420 (free full text).
- ↑ Umbilical cord blood donation: Help without risk , accessed on September 2, 207.
- ^ What health costs ( Memento from December 12, 2013 in the Internet Archive ). In: fit! , No. 4, 2013, p. 25.
- ↑ newswise November 1, 2011
- ↑ The Lancet Oncology, October 2012: Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomized phase 3 trial