Ibuprofen
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
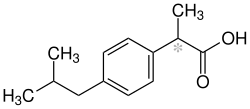
|
||||||||||||||||||||||
| Mixture of stereoisomers - structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ibuprofen | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 13 H 18 O 2 | |||||||||||||||||||||
| Brief description |
white, almost odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
nonselectively inhibits cyclooxygenases I and II |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 206.28 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.175 g cm −3 |
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| boiling point |
154–157 ° C (5 hPa) |
|||||||||||||||||||||
| Vapor pressure |
1.2 m Pa (25 ° C) |
|||||||||||||||||||||
| solubility |
practically insoluble in water: 21 mg l −1 (25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ibuprofen is a drug from the group of non-steroidal anti-inflammatory drugs (NSAIDs), which is used to treat pain, inflammation and fever. Chemically it belongs to the group of aryl propionic acids. The name is - derived from the structure - with a changeover: 2- (4- I so bu tyl phen yl) per propionic acid .
history
The discovery of ibuprofen was the result of a research project at The Boots Pure Drug Company Ltd. under Stewart Adams in the 1950s and 1960s. The aim of the project was to develop new drugs for the treatment of rheumatic diseases. Acetylsalicylic acid was chosen as a model , as it was considered to be the substance with the fewest side effects among the standard therapeutic agents used at the time, such as glucocorticoids and phenylbutazone . First attempts with salicylic acid and its related phthalic acid derivatives led to effective, but clearly more toxic substances. For this purpose, findings on the structure-activity relationship , such as the importance of the carboxylic acid group , were found. Based on this knowledge, the search for new anti-inflammatory substances was extended to other groups of carboxylic acid compounds. A group of phenoxyalkanoic acids originally developed by Boots as herbicides proved particularly promising in preclinical tests in 1958 . Despite positive results in animal experiments , they turned out to be clinically ineffective. The breakthrough came with the phenylalkanoic acids synthesized by John Nicholson at Boots. These agents, including ibuprofen, 1961 as anti-inflammatory agents for patent filed. Three substances with a phenylacetic acid partial structure were first clinically tested . Two of the tested substances led to rash , the third, ibufenac turned out after prolonged use postmarketing as hepatotoxic . Ibuprofen, which was initially not clinically tested due to safety concerns, was shown to be effective and safe in initial trials in 1966 on patients with rheumatoid arthritis at a daily dose of 300 to 600 mg.
In 1969 ibuprofen was launched in the UK under the brand name Brufen with a recommended daily dose of 600 to 800 mg. In the beginning, the results of the treatment turned out to be disappointing, whereupon, after further clinical studies, the daily dose was increased to initially 1200 mg and later to the daily dose of 1200 to 2400 mg used today. In the United States, Ibuprofen was introduced by Upjohn in 1974 under the brand name Motrin with a daily dose of 1200 to 3200 mg.
After an application that initially failed in 1979, ibuprofen was first released from medical prescription requirements in Great Britain in 1983 with a single dose of up to 200 mg and a daily dose of up to 1200 mg . A year later, it also became prescription-free in the US with a daily dose of up to 1600 mg. In Germany, ibuprofen has been available in a single dose of up to 200 mg since 1989 and since 1998 also in up to 400 mg for the oral treatment of pain and fever up to a maximum daily dose of 1200 mg in pharmacies without a doctor's prescription.
presentation
The synthesis route developed by Boots runs over a total of six stages and is based on isobutylbenzene . This is first converted to the ketone in a Friedel-Crafts acylation with acetic anhydride , followed by a Darzens glycidate condensation with ethyl chloroacetate to form the epoxide . Hydrolysis and decarboxylation lead to the aldehyde , which is first converted with hydroxylamine to the oxime , then to the nitrile and finally hydrolyzed to the free acid.
A more recent synthetic route also starts from isobutylbenzene, which is acylated in the first step, but here the ketone is reduced to alcohol with Raney nickel and hydrogen and then carbonylated directly to the product with palladium catalysis.
This synthetic route was awarded the Greener Synthetic Pathways Award for Green Chemistry in 1997 .
In both technical syntheses a racemic mixture of the two enantiomers is obtained.
Stereochemistry
Ibuprofen exists as a 1: 1 mixture ( racemate ) of the pharmacologically active enantiomer ( eutomer ) ( S ) - (+) - ibuprofen ( dexibuprofen ) and the inactive ( R ) - (-) - ibuprofen ( distomer ). ( R ) - (-) - Ibuprofen is converted into ( S ) - (+) - ibuprofen in the body by an isomerase (2-arylpropionyl-CoA-epimerase) . This isomerization is unidirectional; That is, there is only a conversion of ( R ) - (-) - ibuprofen into ( S ) - (+) - ibuprofen, not the other way around. The use of the cheaper racemate ( RS ) - (±) -Ibuprofen as a drug does not seem to be disadvantageous. However, only 50–60% of the applied amount of the distomer is isomerized in this way. In addition, the isomerization proceeds very slowly. Another part of the ( R ) - (-) - ibuprofen is stored in the adipose tissue and dissolved again with a half-life of days. Clinical studies have shown that 200 mg ( S ) - (+) - ibuprofen are about as effective as 400 mg ( RS ) - (±) -ibuprofen.
| Ibuprofen enantiomers | |
|---|---|
 ( R ) -Ibuprofen |
 ( S ) -Ibuprofen |
Pharmacodynamics
Ibuprofen nonselectively inhibits the cyclooxygenases I and II (COX-1 and COX-2), which are responsible for the formation of inflammatory prostaglandins in the organism . This results in the effects of ibuprofen: It relieves pain ( analgesic ), anti-inflammatory ( anti-inflammatory ) and antipyretic ( antipyretic ) and inhibit mucus production in the stomach with the consequence of increased gastric mucosal damage.
Pharmacokinetics
The plasma half-life is about two to three hours. In lower doses (200 to 400 mg for adults) ibuprofen has an analgesic and fever lowering effect, in higher doses (up to 800 mg for adults) it is also anti-inflammatory. About two thirds of the excretion takes place via the kidneys and about one third via the liver. Most inactive metabolites are excreted. The bioavailability and effectiveness of ibuprofen can be increased with piperine , an ingredient in pepper.
Analytics
The reliable qualitative and quantitative determination of ibuprofen and its metabolites in a wide variety of test items is possible after appropriate sample preparation by coupling chromatographic methods with mass spectrometry . The enantiomers of ibuprofen can also be separated and quantified using enantioselective methods. Even with ecotoxicological questions such. B. the investigation of benthic organisms, these methods can be used.
application
application areas
The areas of application are generally for pain therapy such as rheumatoid arthritis , pain in the muscles and the musculoskeletal system, acute gout , headache and toothache, acute menstrual cramps , for lowering fever and especially in children for the treatment of a hemodynamically effective open ductus arteriosus Botalli Premature babies before the 34th week of pregnancy. For cystic fibrosis, high-dose treatment significantly improves symptoms in children with mild cystic fibrosis. However, the potential side effects prevent its widespread use.
Method of application and dosage
Ibuprofen can be administered orally , rectally , dermally , topically, or intravenously . It is dosed depending on age and body weight. When administered orally, ibuprofen 800 mg is recommended as a maximum single dose and between 1200 and 2400 mg as a total daily dose for adults and adolescents aged 15 and over. It is available in Germany without a prescription up to a single dose of 400 mg. A daily dose of 800 mg to 1200 mg is recommended for pain relief, and a daily dose of 1200 mg to 2400 mg for anti-inflammatory purposes. The initial concentration in the blood lasts for about two and a half hours and then decreases. A dose adjustment is made, among others, in patients with severe hepatic impairment and in children.
Side effects
Gastrointestinal symptoms such as heartburn , nausea or diarrhea can occur frequently (1 to 10%) to very often (> 10%) . The occurrence of gastrointestinal bleeding , gastric ulcer or inflammation of the gastric mucous membrane ( gastritis ) as well as gastric perforations, also with fatal outcome, is occasionally observed and depends on the dose and the duration of use. These undesirable side effects are more common in elderly patients.
In the case of inflammatory bowel disease ( Crohn's disease , ulcerative colitis ), ibuprofen can cause relapses. Hypersensitivity reactions such as skin rash or itchy skin ( pruritus ) are possible.
The influence of ibuprofen on blood coagulation is comparatively small; it inhibits platelet function and thus blood coagulation less than acetylsalicylic acid . Nevertheless, the risk of bleeding after surgery can increase. In cases where ibuprofen changes the stomach lining in an inflammatory manner, the anticoagulant effect caused by the drug can lead to blood seeping out of the stomach wall in an uncontrolled manner over a long period of time.
The use of ibuprofen is contraindicated in severe kidney or liver dysfunction .
Edema (e.g. bone marrow edema ) is a known side effect of many painkillers that are based on an inhibition of prostaglandin synthesis, as is also known with ibuprofen.
In association with alcohol , unpredictable side effects and interactions can occur. The combination is therefore not recommended or a lower upper limit of 0.1 to 0.2 liters of wine or 0.25 to 0.5 liters of beer is recommended.
Incidentally, there have been isolated reports of agranulocytosis (severe, life-threatening reduction in granulocytes).
Ibuprofen can have more severe side effects, especially if given for a long time. After long-term administration, the risk of gastrointestinal bleeding increases by a factor of four. In addition, high doses increase the risk of cardiovascular events by a factor of 2.2.
Interactions with other drugs
- Anticoagulants and thrombolytics : Ibuprofen causes a reversible inhibition of platelet aggregation . The platelets are important for blood clotting (wound closure). Anticoagulants also have a negative effect on blood clotting. Thrombolytics break down blood clots (for example in a blocked coronary artery). If ibuprofen is taken together with drugs from one of these drug groups, the risk of bleeding is greater.
- Lithium : Ibuprofen increases the plasma concentration of lithium by reducing its excretion in the kidney. It can thus contribute to lithium poisoning ( intoxication ).
- Acetylsalicylic acid : If ibuprofen is taken at the same time, the anticoagulant effect of acetylsalicylic acid can be reduced. The effect of acetylsalicylic acid (ASA) on the function of platelet aggregation is based on the irreversible inhibition of an enzyme in the platelets, cyclooxygenase-1 (COX-1). The COX-1 in the platelets mainly forms thromboxane -A 2 (TXA 2 ), which activates platelet aggregation via the thromboxane receptor on the platelet surface . Acetylsalicylic acid acetylates (releasing its acetyl residue ) the center of a serine of the COX-1 enzyme irreversibly. However, if ibuprofen is taken at the same time or too soon, both molecules compete for access to the center of the COX-1 enzyme, with ibuprofen holding the upper hand. However, since acetylsalicylic acid is inactivated more quickly than ibuprofen, acetylsalicylic acid is no longer present once the ibuprofen blood level has dropped. The remaining salicylic acid, a breakdown product of acetylsalicylic acid, cannot perform acetylation, so that as a result there is an increasing loss of platelet aggregation inhibition.
- Zinc can interact with NSAIDs such as ibuprofen and reduce the absorption and effectiveness of ibuprofen.
Drug market
Ibuprofen is sold in the form of tablets, capsules, ointments, suppositories, granules to be dissolved in water and children's juices. Tablets and 400 mg (for acute use), ointments, gels, suppositories and partly Juices for children (approved in Germany at present for children aged six months) to treat fever and pain subject in Germany the pharmacies only and can be purchased without a prescription. Preparations in higher doses (600 mg and 800 mg) and preparations for the treatment of inflammation and rheumatic diseases are subject to medical prescription . In some countries (for example in the United States , Poland , the Netherlands , Norway or the United Kingdom ) self-service sales of ibuprofen are permitted in supermarkets, sometimes with restricted quantities .
Since the ibuprofen patent has been free for years, there are numerous generics for the original Brufen , such as the following monopreparations: Aktren (D, A), Alges-X (CH), Algifor-L Forte 400 (CH), Anco (D ), Dismenol (D, A, CH), Dolgit (D, A), Dolocyl (CH), Dolormin Extra (D), Esprenit (D), Eudorlin Extra (D), Grefen (CH), Gyno-Neuralgin (D ), Ibuflam (D), IbuHEXAL (D), Ibumetin (A), Ibutop (D), Ibubeta (D), Irfen (CH), Kontagripp (D), Migraine Ibuprofen (D), Movone (A), Neuralgin extra (D), Nurofen (D, A), Opturem (D), RatioDolor akut (A), Saridon (CH), Spedifen (A, CH), Spalt soft capsules (D), Spidifen (D), Tispol (D), Treupel (CH), Urem (D).
Some ibuprofen supplements contain ibuprofen lysinate, a salt made from ibuprofen and the amino acid lysine . This salt is more soluble in the stomach so that it is absorbed more quickly by the body and should therefore lead to a faster onset of action.
For the treatment of a persistent ductus arteriosus (i.e., which in this case continues after birth) , ibuprofen is available as an injection solution with a special approval as an orphan drug (trade name Pedea ).
As of 2018, ibuprofen as a raw material was produced by only six factories worldwide: Suppliers are Hubei Granules-Biocause and Shandong Xinhua from China, Solara and IOLPC from India, and BASF and SI Group from the USA. At the BASF plant in Bishop, Texas, production of the active ingredient was temporarily suspended in June 2018 due to technical problems. In 2021, a new factory in Ludwigshafen should meet European demand. As a result of failures in the production of active ingredients, delivery bottlenecks repeatedly occurred in Germany in 2018 and 2019 for various finished medicinal products.
In 2017, sales of drugs with the active ingredient ibuprofen in Germany totaled 545.57 million DDD . In 2016, ibuprofen preparations had a market share of 57.7 percent of over-the-counter analgesics in Germany.
Veterinary medicine
In Germany and Switzerland, no ibuprofen-based veterinary drugs are currently approved. The use of ibuprofen in food-producing animals is not permitted as it is not listed in any appendix to Regulation (EEC) No. 2377/90 on maximum levels for veterinary drug residues in food . It can only be used on horses if it is entered in the equine passport and a waiting period of six months before slaughter is observed. Compared to the NSAIDs approved for dogs, the administration of ibuprofen has more gastrointestinal side effects.
literature
- John F. Murray, Jay A. Nadel: Murray & Nadel's Textbook of Respiratory Medicine. 4th edition. Saunders, Philadelphia 2005, ISBN 0-7216-0327-0 .
- Thomas Karow, Ruth Lang-Roth: Pharmacology and Toxicology. 18th edition. Self-published, Pulheim 2009, OCLC 553452252 .
Web links
- Entry on ibuprofen at Vetpharm, accessed April 18, 2012.
Individual evidence
- ↑ a b Safety data sheet ibuprofen from Caelo, accessed on May 8, 2017.
- ^ N. Shankland, CC Wilson, AJ Florence, PJ Cox: Acta Crystallographica , Section C: Crystal Structure Communications. 1997, 53, pp. 951-954; doi: 10.1107 / S0108270197003193 .
- ^ A b A. J. Romero, TC Rhodes: Stereochemical Aspects of the Molecular Pharmaceutics of Ibuprofen. In: J Pharm Pharmacol . 1993, 45, pp. 258-262.
- ↑ KK Kanebo; JP 52100438; 1977.
- ↑ KD Ertel, RA Heasley, C. Koegel, A. Chakrabarti, JT Carstensen: J Pharm Sci . 1990, 79, p. 552; doi: 10.1002 / jps.2600790620 .
- ↑ Entry on ibuprofen in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c Datasheet Ibuprofen from Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ↑ a b c d Kim D. Rainsford: Ibuprofen: A Critical Bibliographic Review . CRC Press, 2003, ISBN 0-203-36258-6 , History and Development of Ibuprofen, pp. 1-22 .
- ↑ patent GB971700 : Anti-inflammatory Agents. Applied on February 2, 1961 , published September 30, 1964 , applicant: Boots Pure Drug Company Ltd., inventor: JS Nicholson, SS Adams.
- ↑ Patent US3385886 : Phenyl propionic acids. Filed July 23, 1963 , published May 28, 1968 , Applicant: Boots Pure Drug Company, Inventor: NJ Stuart & AS Sanders.
- ^ Ibuprofen - a case study in green chemistry. (PDF; 70 kB) (PDF)
- ↑ AM Evans: Enantioselective pharmacodynamics and pharmacokinetics of chiral non-steroidal anti-inflammatory drugs. In: European Journal of Clinical Pharmacology . 1992, 42, pp. 237-256. doi: 10.1007 / BF00266343
- ^ Hermann J. Roth, Christa E. Müller, Gerd Folkers: Stereochemistry & Drugs. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1998, ISBN 3-8047-1485-4 , pp. 175-176.
- ↑ Naproxen Sodium: A New Alternative in Self-Medication. In: Pharmazeutische Zeitung online 16/2002.
- ↑ Olaf Carstens: Duden | about | Spelling, meaning, definition, synonyms, origin. In: www.duden.de. Bibliographisches Institut GmbH, accessed on August 24, 2016 .
- ^ Carsten jewelry, Bernd Engels, Tanja Schirmeister, Reinhold Fink: Chemistry for medical professionals. Pearson Studium, ISBN 978-3-8273-7286-4 , p. 411.
- ↑ a b c d e Federal Institute for Drugs and Medical Devices: Sample text for specialist information on ibuprofen (rheumatological indications). ( RTF ; 175 kB) Retrieved June 6, 2010 .
- ↑ S. Venkatesh, KD Durga, Y. Padmavathi, BM Reddy, R. Mullangi: Influence of piperine on ibuprofen induced antinociception and its pharmacokinetics. In: drug research. Volume 61, number 9, 2011, pp. 506-509, doi: 10.1055 / s-0031-1296235 , PMID 22029226 .
- ↑ B. Yilmaz, AF Erdem: Determination of ibuprofen in human plasma and urine by gas chromatography / mass spectrometry. In: Journal of AOAC International . 2014; 97 (2), pp. 415-420, PMID 24830154
- ↑ J. Manso, E. Larsson, J.Å. Jönsson: Determination of 4'-isobutylacetophenone and other transformation products of anti-inflammatory drugs in water and sludge from five wastewater treatment plants in Sweden by hollow fiber liquid phase microextraction and gas chromatography-mass spectrometry. In: Talanta . 2014; 125, pp. 87-93, PMID 24840419
- ↑ S. Ogawa, H. Tadokoro, M. Sato, T. Higashi: Enantioselective determination of ibuprofen in saliva by liquid chromatography / tandem mass spectrometry with chiral electrospray ionization-enhancing and stable isotope-coded derivatization. In: J Pharm Biomed Anal. 2014; 98, pp. 387-392, PMID 24999866
- ↑ L. Pasquini, JF Munoz, MN Pons, J. Yvon, X. Dauchy, X. France, ND Le, C. France-Lanord, T. Görner: Occurrence of eight household micropollutants in urban wastewater and their fate in a wastewater treatment plant. Statistical evaluation. In: Sci Total Environ. 2014; 481, pp. 459-468, PMID 24631609 .
- ↑ A. Berlioz-Barbier, A. Buleté, J. Faburé, J. Garric, C. Cren-Olivé, E. Vulliet: Multi-residue analysis of emerging pollutants in benthic invertebrates by modified micro-Quick-Easy-Cheap-Efficient -Rugged-Safe extraction and nanoliquid chromatography-nanospray-tandem mass spectrometry analysis. In: J Chromatogr A. 2014. pii: S0021-9673 (14) 01478-2, PMID 25287267
- ^ A. Jaffe, IM Balfour-Lynn: Treatment of severe small airways disease in children with cystic fibrosis: alternatives to corticosteroids. In: Pediatric Drugs. 2002, 4 (6), pp. 381-389; PMID 12038874 .
- ↑ JF Chmiel, MW Konstan: Anti-inflammatory medications for cystic fibrosis lung disease: selecting the most appropriate agent. In: Treat Respir Med. 2005, 4 (4), pp. 255-273; PMID 16086599 .
- ↑ Ibu-ratiopharm technical information , as of August 2015
- ↑ Gesundheit.de: Ibuprofen: Side Effects and Dosage , accessed on December 12, 2016.
- ↑ a b c d Federal Institute for Drugs and Medical Devices (BfArM): Sample text for specialist information on Ibuprofen / Ibuprofen-DL-lysinate (only available in pharmacies). (RTF; 222 kB) 2009, accessed on February 24, 2013 .
- ↑ Medication: In this case, alcohol is allowed. Retrieved June 10, 2019 .
- ↑ Ibuprofen. Retrieved June 10, 2019 .
- ↑ Is it safe to mix ibuprofen and alcohol? Retrieved June 10, 2019 .
- ↑ Agranulocytosis after metamizole - very rare, but more often than expected (from the ADR database) (PDF) In: Deutsches Ärzteblatt. Vol. 108, issue 33, August 19, 2011, p. 1758.
- ↑ a b Baron, Ralf, Koppert, Wolfgang, Strumpf, Michael, Willweber-Strumpf, Anne, Springer-Verlag GmbH: Practical pain medicine, interdisciplinary diagnostics - multimodal therapy. 4th edition 2019. Berlin, ISBN 978-3-662-57486-7 . P. 132 f.
- ↑ Francesca Catella-Lawson, Muredach P. Reilly, Shiv C. Kapoor, Andrew J. Cucchiara, Susan DeMarco, Barbara Tournier, Sachin N. Vyas, Garret A. FitzGerald: Cyclooxygenase Inhibitors and the Antiplatelet Effects of Aspirin . In: New England Journal of Medicine . tape 345 , no. 25 , December 20, 2001, pp. 1809-1817 , doi : 10.1056 / NEJMoa003199 .
- ↑ Be careful with ibuprofen with acetylsalicylic acid. In: Pharmaceutical newspaper . PZ No. 28, 2004.
- ↑ Possible Interactions with: Zinc. University of Maryland; Retrieved September 28, 2013.
- ↑ List of orphan drugs approved in Europe , accessed on February 28, 2019.
- ↑ P. Hollstein: Six factories for ibuprofen , apotheke adhoc, June 26, 2018.
- ↑ Painkillers: BASF production of ibuprofen in the USA is suspended for three months . ( handelsblatt.com [accessed August 23, 2018]).
- ↑ Ibuprofen: Now the mega bottleneck is threatening pharmacy ad hoc.
- ↑ BASF invests in ibuprofen
- ↑ C. Ciulli: ( Page no longer available , search in web archives: Ibuprofen: Now there is a quota ) November 5, 2018.
- ↑ D. Moll: Ibuprofen continues to cause problems . In: Deutsche Apothekerzeitung online . September 2, 2019 ( deutsche-apotheker-zeitung.de ).
- ↑ Sales of leading drugs with the active ingredient ibuprofen in Germany in 2017 , Statista (accessed January 25, 2019)
- ↑ Market share of selected prescription-free analgesics in Germany from 2007 to 2016 , Statista (accessed on January 25, 2019)
- ↑ Julia Nakagawa et al. a .: Side effects from non-approved non-steroidal anti-inflammatory drugs (NSAIDs) in 21 dogs. In: Small Animal Practice. 2010, 55, pp. 364-370.

