Darzens glycidate condensation
The Darzens glycidate condensation is a name reaction in organic chemistry and named after its discoverer, the French chemist Georges Darzens (1867–1954). The reaction is a method for chain extension of aldehydes or ketones with α-chlorocarboxylic acid esters via oxirane carboxylic acid esters.
mechanism
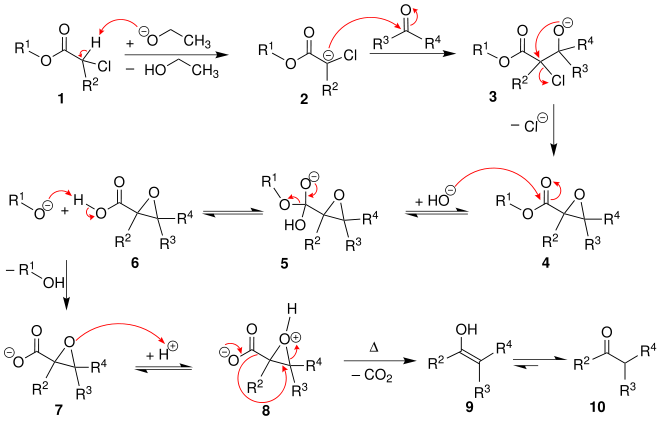
The α-halogen ester 1 is deprotonated and condensed with an aldehyde or a ketone to form an α, β-epoxycarboxylic acid ester 4 . This ester is saponified to form compound 7 and then protonated. After decarboxylation , an aldehyde (R 2 = H) or ketone (R 2 = alkyl, aryl) 10 is formed .
Individual evidence
- ^ Georges Darzens: Méthode générale de synthèse des aldéhydes à l'aide des acidic glycidiques substité . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 139 , 1904, pp. 1214–1217 ( digitized on Gallica ).
- ↑ Georges Darzens: Méthode générale de synthèse d'éthers glycidiques ab substités et de cétones . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 141 , 1905, pp. 766 ( digitized on Gallica ).
- ↑ Georges Darzens: Condensation glycidique des aldéhydes avec l'éther a-chloropropionique . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 142 , 1906, pp. 214–215 ( digitized on Gallica ).
- ^ T. Laue, A. Plagens: Name and keyword reactions of organic chemistry . 5th edition, Teubner Studienbücher Chemie, 2006, ISBN 3-8351-0091-2 , p. 91.