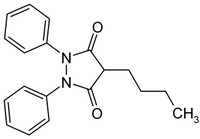
Phenylbutazone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Phenylbutazone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 20 N 2 O 2 | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 308.38 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
105 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Phenylbutazone ( International Nonproprietary Name ), chemically 4-butyl-1,2-diphenylpyrazolidin-dione 3,5- , is a pyrazolidinedione - derivative and is used as a drug from the group of non-steroidal anti-inflammatory drugs having analgesic , antiinflammatory and antipyretic used effect. Phenylbutazone was patented by Geigy (now Novartis ) in 1951.
Applications
Human medicine
In principle, it should only be prescribed for a short time and only when other treatments and newer non-steroidal anti-inflammatory drugs do not work sufficiently. Phenylbutazone is therefore still used today for the treatment of acute pain in inflammatory rheumatic diseases such as
- chronic polyarthritis
- ankylosing spondylitis
- as well as acute gout attacks
Veterinary medicine
Phenylbutazone is commercially available in various dosage forms for oral, intramuscular or intravenous administration, also as an ointment or percutaneous solution. The active ingredient is often used in small and large animal practices. In the European Union , the use of phenylbutazone in food-producing animals is prohibited.
Phenylbutazone is very often used therapeutically in horses. However, phenylbutazone is also misused as a doping agent in equestrian sports. Phenylbutazone tops the statistics in this regard. After the use of the agent in international equestrian sport was banned in the 1990s, this ban was overturned at the FEI General Assembly in 2009 and the amount of 8 micrograms per milliliter of plasma introduced as a new limit value. This means that the new limit is around three times as high as it was before the substance was banned. Due to massive protests, especially from Europe, the introduction of the new regulations was initially postponed to April 2010, and later until the next FEI General Assembly in November 2010. Until then, the list of permitted / prohibited medications should be checked again. On the list issued by the FEI for 2013, phenylbutazone is still only listed as a controlled substance and not as a banned substance .
Analytics
The reliable qualitative and quantitative detection of phenylbutazone is achieved through the use of HPLC coupled with mass spectrometry after suitable sample preparation .
Pharmacokinetics
Phenylbutazone is almost completely absorbed in the gastrointestinal tract. It is mainly absorbed in the small intestine . Maximum blood levels are reached in 1–2 hours after oral administration. The plasma protein binding of the drug is more than 95%, it is ultimately responsible for the long retention time in the body. The metabolism takes place in the liver. Oxyphenbutazone is formed as an effective metabolite . Approx. 70% of the excretion occurs via the kidneys (renal) and approx. 30% via the bile (biliary). Phenylbutazone can cause drug interactions by inducing the cytochrome P450 isoenzyme CYP3A .
Side effects
Phenylbutazone remains in the body for a very long time. Effective concentrations are found in the joints for up to three weeks after stopping use. The active ingredient has very strong anti-inflammatory effects on the one hand, but also very serious side effects on the other. It should therefore only be taken for a few days.
The following side effects are observed: gastrointestinal disorders ( risk of ulcers ), bone marrow damage ( agranulocytosis ), sodium retention ( edema ), increased uric acid excretion and thus the risk of concretion .
Phenylbutazone initially came onto the market in broader application areas. After serious side effects (gastrointestinal damage, blood formation disorders) became known, some of which resulted in death, the indications were restricted in 1984 and further measures were imposed in order to limit the use of phenylbutazone to only what is absolutely necessary. In addition, the approval of fixed combinations of phenylbutazone with corticosteroids for intramuscular injection in severe back pain (example Tomanol B : phenylbutazone, ramifenazone, prednisolone , vitamin B12 , cinchocaine ) was withdrawn in Germany in 1985, because even with short-term use, bleeding in the gastrointestinal tract and gastric mucosa is unacceptably frequent Gastric perforations occurred. In addition, the Tübingen regional council prohibited a pharmaceutical company from advertising the use of a self-made "mixed syringe" of phenylbutazone and vitamin B12 with dexamethasone . The ready-to-use mixture of phenylbutazone with a corticoid for injection is also known as the "Tübinger bomb".
Trade names
- Monopreparations
- Ambene (D), Butagran Equi (A), Equistopar (A), Exrheudon OPT (D), Phenylbutariem for horses and ponies (A). Butazolidine (S)
- Combination preparations
- Ambene parenteral (with lidocaine ) (D)
- historical combinations with corticoids (until 1985): tomanol B (D), dexa-attritin (D)
- Phen-Pred tablets for dogs (with prednisone ) (A)
literature
- DE Gunson et al.: Renal papillary necrosis in horses after phenylbutazone and water deprivation. In: Vet Pathol. 20/1983, pp. 603-610, doi: 10.1177 / 030098588302000512 .
- ME Hough et al: Ulceration and stricture of the right dorsal colon after phenylbutazone administration in four horses. In: Aust Vet J. 77/1999, pp. 785-788, doi: 10.1111 / j.1751-0813.1999.tb12945.x .
- CG MacAllister: Effects of toxic doses of phenylbutazone in ponys. In: Am J Vet. Res. 44/1983, pp. 2277-2279, doi: 10.1016 / S0737-0806 (84) 80053-2 .
- DC Plumb: Veterinary Drug Handbook. PharmaVet Publishing. White Bear Lake, 1991, pp. 40-43.
- DH Snow et al .: Phenylbutazone toxicosis in equidae: a biochemical and pathophysiological study. In: Am J Vet Res. 42/1981, pp. 1754-1759, PMID 7325437 .
- JL Traub et al: Phenylbutazone toxicosis in the foal. In: Am J Vet Res. 44/1983, pp. 1410-1418, PMID 6625291 .
Individual evidence
- ↑ a b c d e Entry on phenylbutazone. In: Römpp Online . Georg Thieme Verlag, accessed on March 31, 2013.
- ↑ a b c Datasheet Phenylbutazone from Sigma-Aldrich , accessed on March 2, 2019 ( PDF ).
- ↑ a b Entry on phenylbutazone at Vetpharm, accessed on June 23, 2013.
- ^ Doping in equestrian sport
- ↑ Article FEI Annual Meetings Copenhagen DEN of November 20, 2009, accessed on the same day ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ Article FEI postpones implementation of “Progressive List” from December 2, 2009, accessed on the same day
- ↑ Article FEI wants to decide on medications from December 18, 2009
- ^ FEI list for 2013 ( Memento from January 20, 2013 in the Internet Archive ) (PDF; 773 kB).
- ↑ Y. You, CE Uboh, LR Soma, F. Guan, X. Li, JA Rudy, J. Chen: Screening, quantification, and confirmation of phenylbutazone and oxyphenbutazone in equine plasma by liquid chromatography-tandem mass spectrometry. In: J Anal Toxicol. 33 (1), Jan-Feb 2009, pp. 41-50. PMID 19161668 .
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (22 July) 2019, pp. 508-517, pp. 510 f.
- ↑ Pharmaceutical Newspaper No. 42, 1969, p. 1530 .
- ↑ Mixture of butazone and cortisone: a publicized malpractice . arznei-telegram 1990, No. 12, p. 107.
- ↑ No diclofenac corticosteroid injections. drug telegram 2000; Vol. 31, No. 10, p. 85.